KIT FOR THE PREPARATION OF TECHNETIUM TC99M DISOFENIN- hepatolite injection, powder, lyophilized, for solution
Pharmalucence, Inc.
----------
HEPATOLITE® Kit for the Preparation of Technetium Tc99m Disofenin for Injection FOR DIAGNOSTIC USE
DESCRIPTION
Each vial contains a sterile, non-pyrogenic, lyophilized mixture of
Disofenin - 20 mg
Stannous Chloride, minimum (SnCL2•2H20) - 0.24mg
Total Tin, maximum (SnCL2•2H20) - 0.6 mg
Prior to lyophilization the pH is adjusted to between 4-5 with HCL and/or NaOH. The contents of the vial are lyophilized and stored under nitrogen.
The drug is administered by intravenous injection for diagnostic use after reconstitution with sterile, non-pyrogenic, oxidant-free Sodium Pertechnetate Tc99m Injection.
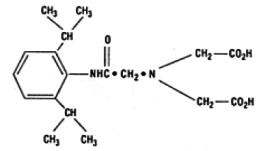
The structure of disofenin is shown below:
The precise structure of stannous technetium-disofenin complex is unknown at this time.
PHYSICAL CHARACTERISTICS
Technetium Tc99m decays by isomeric transition with a physical half-life of 6.02 hours.1 Photons that are useful for detection and imaging studies are listed in Table 1.
|
Table 1. Principal Radiation Emission Data |
||
|
Radiation |
Mean % per Disintegration |
Mean Energy (keV) |
|
Gamma-2 |
89.07 |
140.5 |
1Kocher, David C., Radioactive Decay Data Tables, DOE/TIC-11026, 108 (1981).
External Radiation
The specific gamma ray constant for Tc99m is 5.4 microcoulombs/kg-MBq-hr (0.78R/mCi-hr) at 1cm. The first half value layer is 0.017cm of Pb. A range of values for the relative attenuation of the radiation emitted by this radionuclide that results from interposition of various thicknesses of lead (Pb) is shown in Table 2. To facilitate control of the radiation exposure from MBq (mCi) amounts of this radionuclide, the use of a 0.25cm thickness of lead (Pb) will attenuate the radiation emitted by a factor of 1,000.
|
Table 2. Radiation Attenuation by Lead Shielding |
|
|
Shield Thickness (Pb) cm |
Coefficient of Attenuation |
|
0.017 |
0.5 |
|
0.08 |
10-1 |
|
0.16 |
10-2 |
|
0.25 |
10-3 |
|
0.33 |
10-4 |
To correct for physical decay of this radionuclide, the fractions that remain at selected intervals after the time of calibration are shown in Table 3.
|
Table 3. Physical Decay Chart; Tc 99m Half-Life 6.02 Hours |
|||
|
Hours |
Fraction Remaining |
Hours |
Fraction Remaining |
|
0* |
1.000 |
7 |
.447 |
|
1 |
.891 |
8 |
.398 |
|
2 |
.794 |
9 |
.355 |
|
3 |
.708 |
10 |
.316 |
|
4 |
.631 |
11 |
.282 |
|
5 |
.562 |
12 |
.251 |
|
6 |
.501 |
*Calibration time
CLINICAL PHARMACOLOGY
Disofenin (Hepatolite) is an iminodiacetic acid derivative with no known pharmacologic actions at the doses recommended.
Technetium Tc99m Disofenin is rapidly cleared from the circulation of normal individuals following intravenous administration; about 8% of the injected activity remains in the circulation 30 minutes post-injection. About 9% of the administered activity is excreted in the urine over the first two hours post-injection. The remainder of the activity is essentially quantitatively cleared through the hepatobiliary system. In fasting normal individuals, peak liver uptake occurs by 10 minutes post-injection and peak gallbladder accumulation by 30-40 minutes post-injection. Gallbladder visualization and visualization of intestinal activity occurs by 60 minutes post-injection in individuals with normal hepatobiliary function.
As the serum bilirubin level increases, the blood clearance becomes progressively delayed, resulting in higher background-to-liver radioactivity ratios. Kidney visualization becomes progressively prolonged compared with that in patients with normal biliary excretion. In patients with hepatocellular disease or partial biliary obstruction, there is delayed transit of the radiopharmaceutical to the gut.
Morphine administration has been reported to be effective in shortening the 4 hours usually required for observations, to 90 minutes. In patients who do not have acute cholecystitis, the use of morphine may result in visualization of the gall bladder within an additional 30 minutes.
False negative visualization of the gallbladder in cases of acute cholecystitis, and false positive failure of a normal gallbladder to visualize have been reported with both morphine augmentation and the standard 4 hours of observation.
INDICATIONS AND USAGE
Technetium Tc99m Disofenin is indicated as a hepatobiliary imaging agent. Hepatolite is indicated in the diagnosis of acute cholecystitis as well as to rule out the occurrence of acute cholecystitis in suspected patients with right upper quadrant pain, fever, jaundice, right upper quadrant tenderness and mass or rebound tenderness, but not limited to these signs and symptoms.
In otherwise healthy individuals, non-visualization of the gallbladder 4 hours after administration of Hepatolite following a 2-6 hour fast and in the presence of activity in the small intestine is indicative of a diagnosis of acute cholecystitis. Under the same conditions in an otherwise healthy person, visualization of the gallbladder during a 1 hour scintigraphy is effective in excluding a diagnosis of acute cholecystitis. If the gallbladder is not visualized by 1 hour, scanning must continue for four hours or until the gallbladder is visualized.
When the gall bladder fails to appear within the first 60 minutes of imaging
after the administration of Hepatolite, a single 0.04mg/kg dose of intravenous morphine, diluted in 10mL normal saline may be administered over 3 minutes. (See CLINICAL PHARMACOLOGY section)
PRECAUTIONS
The preparation contains no bacteriostatic preservative. Technetium Tc99m Disofenin should be used within six hours of preparation.
In cases where there has been no visualization of the gallbladder after 60 minutes of scanning, morphine may be carefully administered provided there is no contraindication to the use of narcotics. There should be clear evidence of patency of the common duct, such as observed entry of radiopharmaceutical into the small bowel, prior to the administration of morphine to such patients.
Morphine augmentation has not been associated with any serious adverse events in the reported cases, but the administration of morphine in biliary colic may increase patient discomfort, and the recommended dose of 0.04mg/kg (2-4mg) may be associated with significant respiratory depression and/or postural syncope in vulnerable patients.
Facilities using morphine augmentation should be able to monitor patients for the adverse effects of narcotics and have the means at hand to manage them, including the ready availability of a specific narcotic antagonist such as naloxone.
Cholescintigraphy is only partially effective in the diagnosis or excluding the diagnosis of acute cholecystitis in other conditions such as trauma, intercurrent disease, total parenteral nutrition (TPN) and nothing by mouth (NPO) status, all of which frequently result in false positive results (non-visualization). False negatives (visualization) are rarely seen in certain patients with cholelithiasis (myriad of small stones).
General
Contents of the vial are intended for reconstitution with Technetium Tc99m and are not to be administered directly to patient.
Radioactive drugs must be handled with care and appropriate safety measures should be used to minimize radiation exposure to clinical personnel. Also care should be taken to minimize radiation exposure to the patients consistent with proper patient management.
The contents of the kit before preparation are not radioactive. However, after the Sodium Pertechnetate Tc99m Injection is added, adequate shielding of the final preparation must be maintained.
The components of the kit are sterile and non-pyrogenic. It is essential to follow directions carefully and to adhere to strict aseptic procedures during preparation.
The technetium Tc99m labeling reactions involved in preparing the agent depend on maintaining the stannous ion in the reduced state. Any oxidant present in the sodium pertechnetate Tc99m solution may thus adversely affect the quality of the radiopharmaceutical. Hence, sodium pertechnetate Tc99m solution containing oxidants should not be employed.
Radiopharmaceuticals should be used only by physicians who are qualified by training and experience in the safe use and handling of radionuclides.
Carcinogenesis, Mutagenesis, Impairment of Fertility
No long-term animal studies have been performed to evaluate carcinogenic potential or whether Technetium Tc99m Disofenin affects fertility in males or females.
Pregnancy
Animal reproductive and teratogenicity studies have not been conducted with Technetium Tc99m Disofenin. It is also not known whether Technetium Tc99m Disofenin can cause fetal harm when administered to a pregnant woman or can affect reproductive capacity. There have been no studies in pregnant women. Technetium Tc99m should be given to a pregnant woman only if clearly needed.
Nursing Mothers
Technetium Tc99m is excreted in human milk during lactation. Therefore, formula feedings should be substituted for breast feeding.
Geriatric Use
Clinical studies of Hepatolite did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
ADVERSE REACTIONS
Itching at the site of injection progressing to erythema multiforme has been reported following single administration. Rare cases of chills and nausea have been reported with related compounds. Infrequently, death has been reported in association with the use of this class of agents.
DOSAGE AND ADMINISTRATION
The suggested dose range for i.v. administration, to be employed in the average patient (70kg) is:
Non-Jaundiced patient: 37 - 185 MBq (1-5 mCi)
Patient with serum bilirubin level greater than 5 mg/dL: 111 - 296 MBq (3-8 mCi)
The patient dose should be measured by a suitable radioactivity calibration system immediately prior to patient administration. (If blood is drawn into the syringe, any unnecessary delay prior to injection may lead to clot formation in situ.) Do not backflush the syringe; slow injection is recommended. Radiochemical purity should be checked prior to patient administration.
Store at controlled room temperature (20-25°C) before and after reconstitution.
The patient should be in a fasting state; 4 hours is preferable. False positives (non-visualization) may result if the gallbladder has been emptied by ingestion of food.
This preparation is usually administered to a patient only once. However, should a second dose be required, the interval between doses should not be less than 24 hours.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration whenever solution and container permit.
INSTRUCTIONS FOR PREPARATION OF TECHNETIUM Tc99m DISOFENIN
Preparation of Technetium Tc99m Disofenin from Kit for the Preparation of Technetium Tc99m Disofenin is done by the following aseptic procedure:
a. Prior to adding the Sodium Pertechnetate Tc99m Injection to the vial, write the estimated activity, date, and time of preparation in the space provided on the vial label. Then tear off a radiation symbol and attach it to the neck of the vial.
b. Waterproof gloves should be worn during the preparation procedure. Remove the central plastic disk from the vial and swab the top of the vial closure with alcohol to sanitize the surface.
c. Place the reaction vial in a suitable radiation shield with a fitted radiation shield cap.
d. With a sterile, shielded syringe, aseptically obtain and add to the reaction vial 4-5 ml of sterile, non-pyrogenic, additive-free Sodium Pertechnetate Tc99m Injection containing 444MBq to 3.7GBq (12 to 100mCi) of Tc99m. Note: If Sodium Pertechnetate Tc99m Injection must be diluted for use with Hepatolite, only preservative free sodium chloride injection, USP should be used. Be sure to maintain a nitrogen atmosphere in the vial by not introducing air during reconstitution.
e. Place the radiation shield cap on the vial shield and swirl the contents of the vial for one minute and let stand for 4 minutes.
f. Maintain adequate shielding during the life of the product by using the radiation vial shield with the radiation shield cap in place and by using a syringe shield for withdrawing and injecting the preparation.
g. Using proper shielding, the vial containing the reconstituted solution should be visually inspected to insure that it is free of particulate matter and discoloration prior to injection.
h. Assay the product in a suitable dose calibrator, then complete and affix the “radioactive contents” label to the vial shield.
i. Aseptically withdraw material for use within six (6) hours. Store reconstituted vial at controlled room temperature (20°-25°C). The vial contains no preservative.
RADIATION DOSIMETRY
The estimated absorbed radiation doses2 to organs and tissues of an average patient (70kg) from an intravenous injection of Technetium Tc99m Disofenin are shown in Tables 4 and 5.
|
Table 4. Estimated Radiation Absorbed Doses For Non-Jaundiced Patients |
||
|
Organ |
mGy/185MBq |
(rads/5mCi) |
|
Total Body |
0.7 |
(0.07) |
|
Liver |
1.5 |
(0.15) |
|
Gallbladder Wall* |
7.0 |
(0.7) |
|
Small Intestine |
8.9 |
(0.9) |
|
Upper Large Intestine Wall |
17.4 |
(1.75) |
|
Lower Large Intestine Wall |
12.2 |
(1.25) |
|
Urinary Bladder Wall |
4.4 |
(0.45) |
|
Ovaries |
3.9 |
(0.39) |
|
Testes |
0.3 |
(0.03) |
|
Red Marrow |
0.8 |
(0.08) |
*This value assumes that 80% of the activity localizes in the liver, and that 10% of the liver activity is cleared to the gallbladder.
|
Table 5. Estimated Radiation Absorbed Doses For Jaundiced Patients** |
||
|
Organ |
mGy/296MBq |
(rads/8mCi) |
|
Total Body |
1.2 |
(0.12) |
|
Liver |
5.0 |
(0.5) |
|
Gallbladder Wall |
11.2 |
(1.12) |
|
Small Intestine |
13.0 |
(1.3) |
|
Upper Large Intestine Wall |
26.0 |
(2.6) |
|
Lower Large Intestine Wall |
18.0 |
(1.8) |
|
Urinary Bladder Wall |
7.0 |
(0.7) |
|
Ovaries |
6.0 |
(0.6) |
|
Testes |
0.5 |
(0.05) |
|
Red Marrow |
1.2 |
(0.12) |
**Based on kinetic data for jaundiced patients with malignant obstructive disease.
2 April 1989. Oak Ridge Assoc.Universities.
HOW SUPPLIED
HEPATOLITE® Kit for the Preparation of Technetium Tc99m Disofenin for Injection is supplied as a set of five or thirty vials, sterile and non-pyrogenic.
Prior to lyophilization the pH is adjusted to between 4-5 with hydrochloric acid and/or sodium hydroxide solution. The contents of the vial are lyophilized and stored under nitrogen. Store at controlled room temperature (20 - 25°C) before and after reconstitution. Protect from light. The lyophilized drug product is light sensitive. Technetium Tc99m Disofenin contains no preservatives. Included in each five (5) vial kit is one (1) package insert and twelve (12) radiation labels. Included in each thirty (30) vial kit is one (1) package insert and seventy-two (72) radiation labels.
This reagent kit for the preparation of a radiopharmaceutical is approved for use by persons licensed pursuant to Section 120.547, Code of Massachusetts Regulation 105,
or under equivalent license of the U.S. Nuclear Regulatory Commission or an Agreement State.
Manufactured by:
Pharmalucence, Inc.
Billerica, MA 01821
PL-000027
Rev 1.0
Jun 2018
For ordering Tel. Toll Free: 800-221-7554
(For International, call: 781-275-7120)
PACKAGE LABEL - PRINCIPAL DISPLAY PANEL - 5 VIAL CONTAINER (10 mL)
NDC 45567-0475-1
Sterile, Non-Pyrogenic, Diagnostic Agent for Intravenous Use
Hepatolite®
Kit for the Preparation of Technetium Tc99m Disofenin for Injection
Contents and Storage Conditions:
Disofenin - 20mg
Stannous Chloride, minimum (SnCL2•2H20) - 0.24mg
Total Tin, maximum (SnCL2•2H20) - 0.6 mg
The pH is adjusted with HCL and/or NaOH
Store at controlled room temperature (20° - 25°C). Protect from light
Lyophilized and stored under nitrogen
Rx only
Manufactured By:
Pharmalucence, Inc.
Billerica, MA 01821
PL-000028
Rev 0.1
Jun 2012
Tc99m Activity
Time/Date Prepared
See Package Insert for dosage information
Reconstitute with additive-free Tc99m and store at controlled room temperature
Use within 6 hours
CONTAINS NO PRESERVATIVE
Lot No:
Exp. Date:
PACKAGE LABEL - PRINCIPAL DISPLAY PANEL - 5 VIAL CARTON
NDC 45567-0475-1
Hepatolite®
Kit for the Preparation of Technetium Tc99m Disofenin for Injection
Sterile
Non-Pyrogenic
Diagnostic Agent for Intravenous Use
Lyophilized
5 vials
Manufactured By:
Pharmalucence, Inc.
Billerica, MA 01821
PL-000030
Rev 0.1
Jun 2012
CONTENTS AND STORAGE CONDITIONS:
1 Package Insert, 12 Radiation Labels and 5 Vials each containing:
Disofenin - 20mg
Stannous Chloride, minimum (SnCL2•2H20) - 0.24mg
Total Tin, maximum (SnCL2•2H20) - 0.6 mg
The pH is adjusted with HCL and/or NaOH
Store at controlled room temperature (20° - 25°C). Protect from light
CONTAINS NO PRESERVATIVE
See Package Insert for dosage information. Reconstitute with additive-free
Tc99m and store at controlled room temperature (20-25°C). Use within 6 hours
Rx Only
IMPORTANT: Read enclosed Package Insert for full information on preparation, use and indications.
WARNING: Radiopharmaceuticals should be used by persons who are qualified by specific training in the safe use and handling of radionuclides and whose experience and training have been approved by the appropriate governmental agency authorized to license the use of radionuclides.
PACKAGE LABEL - PRINCIPAL DISPLAY LABEL - 30 VIAL CONTAINER (10 mL)
NDC 45567-0475-2
Sterile, Non-Pyrogenic, Diagnostic Agent for Intravenous Use
Hepatolite®
Kit for the Preparation of Technetium Tc99m Disofenin for Injection
Contents and Storage Conditions:
Disofenin - 20mg
Stannous Chloride, minimum (SnCL2•2H20) - 0.24mg
Total Tin, maximum (SnCL2•2H20) - 0.6 mg
The pH is adjusted with HCL and/or NaOH
Store at controlled room temperature (20° - 25°C). Protect from light
Lyophilized and stored under nitrogen
Rx only
Manufactured By:
Pharmalucence, Inc.
Billerica, MA 01821
PL-000028
Rev 0.1
Jun 2012
Tc99m Activity
Time/Date Prepared
See Package Insert for dosage information
Reconstitute with additive-free Tc99m and store at controlled room temperature
Use within 6 hours
CONTAINS NO PRESERVATIVE
Lot No:
Exp. Date:
PACKAGE LABEL - PRINCIPAL DISPLAY LABEL - 30 VIAL CARTON
NDC 45567-0475-2
CONVENIENCE PACK
Hepatolite®
Kit for the Preparation of Technetium Tc99m Disofenin for Injection
STERILE
NON-PYROGENIC
DIAGNOSTIC AGENT FOR
INTRAVENOUS USE
Manufactured By:
Pharmalucence, Inc.
Billerica, MA 01821
PL-000031
Rev 0.1
Jun 2012
For Customer Service call: 1-800-221-7554
Tear along perforations
Ready to dispense
Package Insert and Radiation Labels
inside bottom flap
Rx only
IMPORTANT: Read enclosed Package Insert for full information on preparation use and indications.
WARNING: Radiopharmaceuticals should be used by persons who are qualified by specific training in the safe use and handling of radionuclides and whose experience and training have been approved by the appropriate governmental agency authorized to license the use of radionuclides.
Manufactured By:
Pharmalucence, Inc.
Billerica, MA 01821
For Customer Service call: 1-800-221-7554
Contents & Storage Conditions:
1 Package Insert, 72 Radiation Labels and 30 Vials each containing:
Disofenin - 20mg
Stannous Chloride, minimum (SnCL2•2H20) - 0.24mg
Total Tin, maximum (SnCL2•2H20) - 0.6 mg
The pH is adjusted with HCL and/or NaOH
Store at controlled room temperature (20° - 25°C)
Protect from light.
CONTAINS NO PRESERVATIVE.
See Package Insert for dosage information. Reconstitute with additive-free
Tc99m and store at controlled room temperature (20-25°C). Use within 6 hours.
PACKAGE LABEL – PRINCIPAL DISPLAY PANEL – RADIATION SHIELD
CAUTION:
RADIOACTIVE MATERIAL
Rx Only
Technetium Tc99m Disofenin for Injection
Contents
Disofenin – 20 mg
Stannous Chloride, minimum (SnCl2 · 2H2O) – 0.24mg
Total Tin, maximum (SnCl2 · 2H2O) – 0.6 mg
Use within 6 hours.
___________________________________________
MBq(mCi) Tc99m/ml
___________________________________________
Volume ml
___________________________________________
Time/Date Prepared
___________________________________________
Expiration Time Lot No.
PL-000029
Rev 0.1
Jun 2012
| KIT FOR THE PREPARATION OF TECHNETIUM TC99M DISOFENIN
hepatolite injection, powder, lyophilized, for solution |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Pharmalucence, Inc. (139261648) |
| Registrant - Pharmalucence, Inc. (139261648) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Pharmalucence Inc. | 139261648 | LABEL(45567-0475) , PACK(45567-0475) , ANALYSIS(45567-0475) | |