Label: XOPENEX HFA- levalbuterol tartrate aerosol, metered
-
Contains inactivated NDC Code(s)
NDC Code(s): 54868-5689-0 - Packager: Physicians Total Care, Inc.
- This is a repackaged label.
- Source NDC Code(s): 63402-510
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated May 7, 2012
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
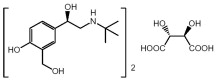
The active component of XOPENEX HFA (levalbuterol tartrate) Inhalation Aerosol is levalbuterol tartrate, the (R)-enantiomer of albuterol. Levalbuterol tartrate is a relatively selective beta2-adrenergic receptor agonist (see CLINICAL PHARMACOLOGY). Levalbuterol tartrate has the chemical name (R)-α1-[[(1,1-dimethylethyl)amino]methyl]-4-hydroxy-1,3-benzenedimethanol L-tartrate (2:1 salt), and it has the following chemical structure:
The molecular weight of levalbuterol tartrate is 628.71, and its empirical formula is (C13H21NO3)2 · C4H6O6. It is a white to light-yellow solid, freely soluble in water and very slightly soluble in ethanol.
Levalbuterol tartrate is the generic name for (R)-albuterol tartrate in the United States. XOPENEX HFA Inhalation Aerosol is a pressurized metered-dose aerosol inhaler (MDI), which produces an aerosol for oral inhalation. It contains a suspension of micronized levalbuterol tartrate, propellant HFA-134a (1,1,1,2-tetrafluoroethane), Dehydrated Alcohol USP, and Oleic Acid NF.
The inhaler should be primed by releasing 4 sprays into the air, away from the face, before using it for the first time and when the inhaler has not been used for more than 3 days. After priming with 4 actuations, each actuation delivers 59 mcg of levalbuterol tartrate (equivalent to 45 mcg of levalbuterol free base) from the actuator (or mouthpiece). Each 15 g canister provides 200 actuations (or inhalations) and each 8.4 g canister provides 80 actuations (or inhalations).
This product does not contain chlorofluorocarbons (CFCs).
-
CLINICAL PHARMACOLOGY
Mechanism of Action: Activation of beta2-adrenergic receptors on airway smooth muscle leads to the activation of adenylate cyclase and to an increase in the intracellular concentration of cyclic-3', 5'-adenosine monophosphate (cyclic AMP). The increase in cyclic AMP is associated with the activation of protein kinase A, which in turn, inhibits the phosphorylation of myosin and lowers intracellular ionic calcium concentrations, resulting in muscle relaxation. Levalbuterol relaxes the smooth muscles of all airways, from the trachea to the terminal bronchioles. Increased cyclic AMP concentrations are also associated with the inhibition of the release of mediators from mast cells in the airways. Levalbuterol acts as a functional antagonist to relax the airway irrespective of the spasmogen involved, thus protecting against all bronchoconstrictor challenges. While it is recognized that beta2-adrenergic receptors are the predominant receptors on bronchial smooth muscle, data indicate that there are beta-receptors in the human heart, 10% to 50% of which are beta2-adrenergic receptors. The precise function of these receptors has not been established (see WARNINGS). However, all beta-adrenergic agonist drugs can produce a significant cardiovascular effect in some patients, as measured by pulse rate, blood pressure, symptoms, and/or electrocardiographic changes.
Preclinical
Results from in vitro studies of binding to human beta-adrenergic receptors demonstrated that levalbuterol has approximately 2-fold greater binding affinity than racemic albuterol and approximately 100-fold greater binding affinity than (S)-albuterol. In guinea pig airways, levalbuterol HCl and racemic albuterol decreased the response to spasmogens (e.g., acetylcholine and histamine), whereas (S)-albuterol was ineffective. These results suggest that the bronchodilatory effects of racemic albuterol are attributable to the (R)-enantiomer.
Intravenous studies in rats with racemic albuterol sulfate have demonstrated that albuterol crosses the blood-brain barrier and reaches brain concentrations amounting to approximately 5.0% of the plasma concentrations. In structures outside the blood-brain barrier (pineal and pituitary glands), racemic albuterol concentrations were found to be 100 times those in the whole brain.
Studies in laboratory animals (minipigs, rodents, and dogs) have demonstrated the occurrence of cardiac arrhythmias and sudden death (with histologic evidence of myocardial necrosis) when beta-agonists and methylxanthines are administered concurrently. The clinical significance of these findings is unknown.
Propellant HFA-134a is devoid of pharmacological activity except at very high doses in animals (380 to 1300 times the maximum human exposure based on comparisons of AUC values), primarily producing ataxia, tremors, dyspnea, or salivation. These are similar to effects produced by the structurally related chlorofluorocarbons (CFCs), which have been used extensively in metered-dose inhalers.
In animals and humans, propellant HFA-134a was found to be rapidly absorbed and rapidly eliminated, with an elimination half-life of 3 to 27 minutes in animals and 5 to 7 minutes in humans. Time to maximum plasma concentration (tmax) and mean residence time are both extremely short, leading to a transient appearance of HFA-134a in the blood with no evidence of accumulation.
Pharmacokinetics
A population pharmacokinetic (PPK) model was developed using plasma concentrations of (R)-albuterol obtained from 632 asthmatic patients aged 4 to 81 years in three large trials. The PPK model-derived pharmacokinetic parameters for (R)-albuterol in pediatric and adolescent/adult patients receiving a 90 mcg dose of XOPENEX HFA (levalbuterol tartrate) Inhalation Aerosol or a 180 mcg dose of racemic albuterol by HFA metered-dose inhaler are presented in Table 1.
These pharmacokinetic data indicate that mean exposure to (R)-albuterol was 13% to 16% less in adult and 30% to 32% less in pediatric patients given XOPENEX HFA Inhalation Aerosol as compared to those given a comparable dose of racemic albuterol. When compared to adult patients, pediatric patients given 90 mcg of levalbuterol have a 17% lower mean exposure to (R)-albuterol.
Table 1: Mean Model-Predicted (R)-Albuterol Pharmacokinetic Parameters Study
PopulationParameter Treatment XOPENEX HFA Inhalation Aerosol Racemic Albuterol HFA MDI Adolescent/Adult Patients
(≥12 years)Cmax (ng/mL) 0.199 0.238 tmax (hr) 0.54 0.53 AUC(0-6) (ng·hr/mL) 0.695 0.798 Pediatric Patients
(4-11 years)Cmax (ng/mL) 0.163 0.238 tmax (hr) 0.76 0.78 AUC(0-6) (ng·hr/mL) 0.579 0.828 Metabolism and Elimination
Information available in the published literature suggests that the primary enzyme responsible for the metabolism of albuterol enantiomers in humans is SULT1A3 (sulfotransferase). When racemic albuterol was administered either intravenously or via inhalation after oral charcoal administration, there was a 3- to 4-fold difference in the area under the concentration-time curves between the (R)- and (S)-albuterol enantiomers, with (S)-albuterol concentrations being consistently higher. However, without charcoal pretreatment, after either oral or inhalation administration the differences were 8- to 24-fold, suggesting that that (R)-albuterol is preferentially metabolized in the gastrointestinal tract, presumably by SULT1A3.
The primary route of elimination of albuterol enantiomers is through renal excretion (80% to 100%) of either the parent compound or the primary metabolite. Less than 20% of the drug is detected in the feces. Following intravenous administration of racemic albuterol, between 25% and 46% of the (R)-albuterol fraction of the dose was excreted as unchanged (R)-albuterol in the urine.
Special Populations
Hepatic Impairment: The effect of hepatic impairment on the pharmacokinetics of XOPENEX HFA Inhalation Aerosol has not been evaluated.
Renal Impairment: The effect of renal impairment on the pharmacokinetics of racemic albuterol was evaluated in 5 subjects with creatinine clearance of 7 to 53 mL/min, and the results were compared with those from healthy volunteers. Renal disease had no effect on the half-life, but there was a 67% decline in racemic albuterol clearance. Caution should be used when administering high doses of XOPENEX HFA Inhalation Aerosol to patients with renal impairment.
Clinical Trials
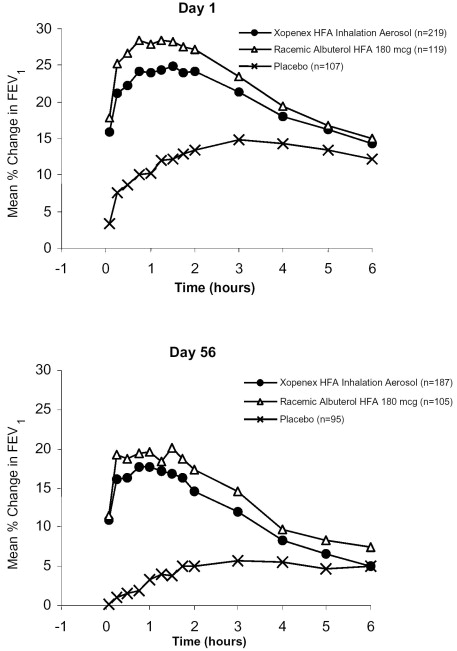
Adults and Adolescents: The efficacy and safety of XOPENEX HFA Inhalation Aerosol were established in two 8-week, multicenter, randomized, double-blind, active- and placebo-controlled trials in 748 adults and adolescents with asthma between the ages of 12 and 81 years. In these two trials, XOPENEX HFA Inhalation Aerosol (403 patients) was compared to an HFA-134a placebo MDI (166 patients), and the trials included a marketed albuterol HFA-134a MDI (179 patients) as an active control. Serial forced expiratory volume in 1 second (FEV1) measurements demonstrated that 90 mcg (2 inhalations) of XOPENEX HFA Inhalation Aerosol produced significantly greater improvement in FEV1 over the pretreatment value than placebo. The results from one of the trials are shown in Figure 1 as the mean percent change in FEV1 from test-day baseline at Day 1 (n=445) and Day 56 (n=387). The results from the second trial were similar.
Figure 1: Percent Change in FEV1 from Test-Day Baseline in Adults and Adolescents Aged 12 to 81 Years at Day 1 and Day 56
For XOPENEX HFA Inhalation Aerosol on Day 1, the median time to onset of a 15% increase in FEV1 ranged from 5.5 to 10.2 minutes and the median time to peak effect ranged from 76 to 78 minutes. In the responder population, on Day 1 the median duration of effect as measured by a 15% increase in FEV1 was 3 to 4 hours, with duration of effect in some patients of up to 6 hours.
Pediatrics: The efficacy and safety of XOPENEX HFA Inhalation Aerosol in children were established in a 4-week, multicenter, randomized, double-blind, active- and placebo-controlled trial in 150 pediatric patients with asthma between the ages of 4 and 11 years. In this trial, XOPENEX HFA Inhalation Aerosol (76 patients) was compared to a placebo HFA-134a MDI (35 patients), and the trial included a marketed albuterol HFA-134a MDI (39 patients) as an active control. Serial FEV1 measurements demonstrated that 90 mcg (2 inhalations) of XOPENEX HFA Inhalation Aerosol produced significantly greater improvement in FEV1 over the pretreatment value than placebo and were consistent with the efficacy findings in the adult studies.
For XOPENEX HFA Inhalation Aerosol, on Day 1 the median time to onset of a 15% increase in FEV1 was 4.5 minutes and the median time to peak effect was 77 minutes. In the responder population, the median duration of effect as measured by a 15% increase in FEV1 was 3 hours, with a duration of effect in some pediatric patients of up to 6 hours.
- INDICATIONS AND USAGE
- CONTRAINDICATIONS
-
WARNINGS
- Paradoxical Bronchospasm: Like other inhaled beta-adrenergic agonists, XOPENEX HFA Inhalation Aerosol can produce paradoxical bronchospasm, which may be life-threatening. If paradoxical bronchospasm occurs, XOPENEX HFA (levalbuterol tartrate) Inhalation Aerosol should be discontinued immediately and alternative therapy instituted. It should be recognized that paradoxical bronchospasm, when associated with inhaled formulations, frequently occurs with the first use of a new canister.
- Deterioration of Asthma: Asthma may deteriorate acutely over a period of hours or chronically over several days or longer. If the patient needs more doses of XOPENEX HFA Inhalation Aerosol than usual, this may be a marker of destabilization of asthma and requires reevaluation of the patient and treatment regimen, giving special consideration to the possible need for anti-inflammatory treatment, e.g., corticosteroids.
- Use of Anti-Inflammatory Agents: The use of a beta-adrenergic agonist alone may not be adequate to control asthma in many patients. Early consideration should be given to adding anti-inflammatory agents, e.g., corticosteroids, to the therapeutic regimen.
- Cardiovascular Effects: XOPENEX HFA Inhalation Aerosol, like other beta-adrenergic agonists, can produce clinically significant cardiovascular effects in some patients, as measured by heart rate, blood pressure, and/or symptoms. Although such effects are uncommon after administration of XOPENEX HFA Inhalation Aerosol at recommended doses, if they occur, the drug may need to be discontinued. In addition, beta-agonists have been reported to produce electrocardiogram (ECG) changes, such as flattening of the T wave, prolongation of the QTc interval, and ST segment depression. The clinical significance of these findings is unknown. Therefore, XOPENEX HFA Inhalation Aerosol, like all sympathomimetic amines, should be used with caution in patients with cardiovascular disorders, especially coronary insufficiency, cardiac arrhythmias, and hypertension.
- Do Not Exceed Recommended Dose: Fatalities have been reported in association with excessive use of inhaled sympathomimetic drugs in patients with asthma. The exact cause of death is unknown, but cardiac arrest following an unexpected development of a severe acute asthmatic crisis and subsequent hypoxia is suspected.
- Immediate Hypersensitivity Reactions: Immediate hypersensitivity reactions may occur after administration of racemic albuterol, as demonstrated by rare cases of urticaria, angioedema, rash, bronchospasm, anaphylaxis, and oropharyngeal edema. The potential for hypersensitivity must be considered in the clinical evaluation of patients who experience immediate hypersensitivity reactions while receiving XOPENEX HFA Inhalation Aerosol.
-
PRECAUTIONS
General
XOPENEX HFA (levalbuterol tartrate) Inhalation Aerosol, like all sympathomimetic amines, should be used with caution in patients with cardiovascular disorders, especially coronary insufficiency, hypertension, and cardiac arrhythmias; in patients with convulsive disorders, hyperthyroidism, or diabetes mellitus; and in patients who are unusually responsive to sympathomimetic amines. Clinically significant changes in systolic and diastolic blood pressure have been seen in individual patients and could be expected to occur in some patients after the use of any beta-adrenergic bronchodilator.
Large doses of intravenous racemic albuterol have been reported to aggravate preexisting diabetes mellitus and ketoacidosis. As with other beta-adrenergic agonist medications, XOPENEX HFA Inhalation Aerosol may produce significant hypokalemia in some patients, possibly through intracellular shunting, which has the potential to produce adverse cardiovascular effects. The decrease is usually transient, not requiring supplementation.
Information for Patients
See illustrated Patient's Instructions for Use. SHAKE WELL BEFORE USING. Patients should be given the following information: It is recommended to prime the inhaler before using for the first time and in cases where the inhaler has not been used for more than 3 days by releasing 4 test sprays into the air, away from the face.
KEEPING THE PLASTIC ACTUATOR CLEAN IS VERY IMPORTANT TO PREVENT MEDICATION BUILD-UP AND BLOCKAGE. THE ACTUATOR SHOULD BE WASHED, SHAKEN TO REMOVE EXCESS WATER, AND AIR-DRIED THOROUGHLY AT LEAST ONCE A WEEK. THE INHALER MAY CEASE TO DELIVER MEDICATION IF NOT PROPERLY CLEANED.
The actuator should be cleaned (with the canister removed) by running warm water through the top and bottom for 30 seconds at least once a week. Do not attempt to clean the metal canister or allow the metal canister to become wet. Never immerse the metal canister in water. The actuator must be shaken to remove excess water, then air-dried thoroughly (such as overnight). Blockage from medication build-up or improper medication delivery may result from failure to clean and thoroughly air-dry the actuator.
If the actuator becomes blocked (little or no medication coming out of the mouthpiece), the blockage may be removed by washing the actuator as described above.
If it is necessary to use the inhaler before it is completely dry, shake excess water off the plastic actuator, replace canister, shake well, test-spray twice away from face, and take the prescribed dose. After such use, the actuator should be rewashed and allowed to air-dry thoroughly.
The action of XOPENEX HFA Inhalation Aerosol should last for 4 to 6 hours. XOPENEX HFA Inhalation Aerosol should not be used more frequently than recommended. Do not increase the dose or frequency of doses of XOPENEX HFA Inhalation Aerosol without consulting your physician. If you find that treatment with XOPENEX HFA Inhalation Aerosol becomes less effective for symptomatic relief, your symptoms become worse, and/or you need to use the product more frequently than usual, you should seek medical attention immediately. While you are using XOPENEX HFA Inhalation Aerosol, other inhaled drugs and asthma medication should be taken only as directed by your physician.
Common adverse effects of treatment with inhaled beta-agonists include palpitations, chest pain, rapid heart rate, tremor, and nervousness. If you are pregnant or nursing, contact your physician about use of XOPENEX HFA Inhalation Aerosol. Effective and safe use of XOPENEX HFA Inhalation Aerosol includes an understanding of the way that it should be administered.
Use XOPENEX HFA Inhalation Aerosol only with the actuator supplied with the product. Discard the canister after 200 sprays have been used from the 15 g canister or after 80 sprays have been used from the 8.4 g canister. Never immerse the canister in water to determine how full the canister is (“float test”).
In general, the technique for administering XOPENEX HFA Inhalation Aerosol to children is similar to that for adults. Children should use XOPENEX HFA Inhalation Aerosol under adult supervision, as instructed by the patient's physician. (See Patient's Instructions for Use.)
Drug Interactions
Other short-acting sympathomimetic aerosol bronchodilators or epinephrine should be used with caution with XOPENEX HFA Inhalation Aerosol. If additional adrenergic drugs are to be administered by any route, they should be used with caution to avoid deleterious cardiovascular effects.
- Beta-blockers: Beta-adrenergic receptor blocking agents not only block the pulmonary effect of beta-adrenergic agonists, such as XOPENEX HFA Inhalation Aerosol, but may produce severe bronchospasm in asthmatic patients. Therefore, patients with asthma should not normally be treated with beta-blockers. However, under certain circumstances, e.g., as prophylaxis after myocardial infarction, there may be no acceptable alternatives to the use of beta-adrenergic blocking agents in patients with asthma. In this setting, cardioselective beta-blockers should be considered, although they should be administered with caution.
- Diuretics: The ECG changes and/or hypokalemia that may result from the administration of non–potassium-sparing diuretics (such as loop and thiazide diuretics) can be acutely worsened by beta-agonists, especially when the recommended dose of the beta-agonist is exceeded. Although the clinical significance of these effects is not known, caution is advised in the coadministration of beta-agonists with non–potassium-sparing diuretics.
- Digoxin: Mean decreases of 16% to 22% in serum digoxin levels were demonstrated after single-dose intravenous and oral administration of racemic albuterol, respectively, to normal volunteers who had received digoxin for 10 days. The clinical significance of these findings for patients with obstructive airway disease who are receiving XOPENEX HFA Inhalation Aerosol and digoxin on a chronic basis is unclear. Nevertheless, it would be prudent to carefully evaluate the serum digoxin levels in patients who are currently receiving digoxin and XOPENEX HFA Inhalation Aerosol.
- Monoamine Oxidase Inhibitors or Tricyclic Antidepressants: XOPENEX HFA Inhalation Aerosol should be administered with extreme caution to patients being treated with monoamine oxidase inhibitors or tricyclic antidepressants, or within 2 weeks of discontinuation of such agents, because the action of albuterol on the vascular system may be potentiated.
Carcinogenesis, Mutagenesis, and Impairment of Fertility
No carcinogenesis or impairment of fertility studies have been carried out with levalbuterol tartrate. However, racemic albuterol sulfate has been evaluated for its carcinogenic potential and ability to impair fertility.
In a 2-year study in Sprague-Dawley rats, racemic albuterol sulfate caused a significant dose-related increase in the incidence of benign leiomyomas of the mesovarium at, and above, dietary doses of 2 mg/kg/day (approximately 30 times the maximum recommended daily inhalation dose of levalbuterol tartrate for adults on a mg/m2 basis and approximately 15 times the maximum recommended daily inhalation dose of levalbuterol tartrate for children on a mg/m2 basis). In another study, this effect was blocked by the coadministration of propranolol, a nonselective beta-adrenergic antagonist. In an 18-month study in CD-1 mice, racemic albuterol sulfate showed no evidence of tumorigenicity at dietary doses up to 500 mg/kg/day (approximately 3800 times the maximum recommended daily inhalation dose of levalbuterol tartrate for adults on a mg/m2 basis and approximately 1800 times the maximum recommended daily inhalation dose of levalbuterol tartrate for children on a mg/m2 basis). In a 22-month study in the Golden hamster, racemic albuterol sulfate showed no evidence of tumorigenicity at dietary doses up to 50 mg/kg/day (approximately 500 times the maximum recommended daily inhalation dose of levalbuterol tartrate for adults on a mg/m2 basis and approximately 240 times the maximum recommended daily inhalation dose of levalbuterol tartrate for children on a mg/m2 basis).
Levalbuterol HCl was not mutagenic in the Ames test or the CHO/HPRT Mammalian Forward Gene Mutation Assay. Levalbuterol HCl was not clastogenic in the in vivo micronucleus test in mouse bone marrow. Racemic albuterol sulfate was negative in an in vitro chromosomal aberration assay in CHO cell cultures.
Reproduction studies in rats using racemic albuterol sulfate demonstrated no evidence of impaired fertility at oral doses up to 50 mg/kg/day (approximately 750 times the maximum recommended daily inhalation dose of levalbuterol tartrate for adults on a mg/m2 basis).
Teratogenic Effects - Pregnancy Category C
A reproduction study in New Zealand White rabbits demonstrated that levalbuterol HCl was not teratogenic when administered orally at doses up to 25 mg/kg/day (approximately 750 times the maximum recommended daily inhalation dose of levalbuterol tartrate for adults on a mg/m2 basis).
However, racemic albuterol sulfate has been shown to be teratogenic in mice and rabbits. A study in CD-1 mice given racemic albuterol sulfate subcutaneously showed cleft palate formation in 5 of 111 (4.5%) fetuses at 0.25 mg/kg/day (approximately 2 times the maximum recommended daily inhalation dose of levalbuterol tartrate for adults on a mg/m2 basis) and in 10 of 108 (9.3%) fetuses at 2.5 mg/kg/day (approximately 20 times the maximum recommended daily inhalation dose of levalbuterol tartrate for adults on a mg/m2 basis). The drug did not induce cleft palate formation when administered subcutaneously at a dose of 0.025 mg/kg/day (less than the maximum recommended daily inhalation dose of levalbuterol tartrate for adults on a mg/m2 basis). Cleft palate also occurred in 22 of 72 (30.5%) fetuses from females treated subcutaneously with 2.5 mg/kg/day of isoproterenol (positive control).
A reproduction study in Stride Dutch rabbits revealed cranioschisis in 7 of 19 (37%) fetuses when racemic albuterol sulfate was administered orally at a dose of 50 mg/kg/day (approximately 1500 times the maximum recommended daily inhalation dose of levalbuterol tartrate for adults on a mg/m2 basis).
A study in which pregnant rats were dosed with radiolabeled racemic albuterol sulfate demonstrated that drug-related material is transferred from the maternal circulation to the fetus.
There are no adequate and well-controlled studies of XOPENEX HFA Inhalation Aerosol in pregnant women. Because animal reproduction studies are not always predictive of human response, XOPENEX HFA Inhalation Aerosol should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
During marketing experience of racemic albuterol, various congenital anomalies, including cleft palate and limb defects, have been rarely reported in the offspring of patients being treated with racemic albuterol. Some of the mothers were taking multiple medications during their pregnancies. No consistent pattern of defects can be discerned, and a relationship between racemic albuterol use and congenital anomalies has not been established.
Use in Labor and Delivery
Because of the potential for beta-adrenergic agonists to interfere with uterine contractility, the use of XOPENEX HFA Inhalation Aerosol for the treatment of bronchospasm during labor should be restricted to those patients in whom the benefits clearly outweigh the risk.
Tocolysis
XOPENEX HFA Inhalation Aerosol has not been approved for the management of preterm labor. The benefit:risk ratio when levalbuterol tartrate is administered for tocolysis has not been established. Serious adverse reactions, including maternal pulmonary edema, have been reported during or following treatment of premature labor with beta2-agonists, including racemic albuterol.
Nursing Mothers
Plasma concentrations of levalbuterol after inhalation of therapeutic doses are very low in humans. It is not known whether levalbuterol is excreted in human milk.
Because of the potential for tumorigenicity shown for racemic albuterol in animal studies and the lack of experience with the use of XOPENEX HFA Inhalation Aerosol by nursing mothers, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother. Caution should be exercised when XOPENEX HFA Inhalation Aerosol is administered to a nursing woman.
Pediatrics
The safety and efficacy of XOPENEX HFA Inhalation Aerosol have been established in pediatric patients 4 years of age and older in an adequate and well-controlled clinical trial (see Clinical Trials). Use of XOPENEX HFA Inhalation Aerosol in children is also supported by evidence from adequate and well-controlled studies of XOPENEX HFA Inhalation Aerosol in adults, considering that the pathophysiology, systemic exposure of the drug, and clinical profile in pediatric and adult patients are substantially similar. Safety and effectiveness of XOPENEX HFA Inhalation Aerosol in pediatric patients below the age of 4 years have not been established.
Geriatrics
Clinical studies of XOPENEX HFA Inhalation Aerosol did not include sufficient numbers of subjects aged 65 and older to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant diseases or other drug therapy.
Albuterol is known to be substantially excreted by the kidney, and the risk of toxic reactions may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function.
-
ADVERSE REACTIONS
Adverse event information concerning XOPENEX HFA (levalbuterol tartrate) Inhalation Aerosol in adults and adolescents is derived from two 8-week, multicenter, randomized, double-blind, active- and placebo-controlled trials in 748 adult and adolescent patients with asthma that compared XOPENEX HFA Inhalation Aerosol, a marketed albuterol HFA inhaler, and an HFA-134a placebo inhaler. Table 2 lists the incidence of all adverse events (whether considered by the investigator to be related or unrelated to drug) from these trials that occurred at a rate of 2% or greater in the group treated with XOPENEX HFA Inhalation Aerosol and more frequently than in the HFA-134a placebo inhaler group.
Table 2: Adverse Event Incidence (% of Patients) in Two 8-Week Clinical Trials in Adults and Adolescents ≥ 12 Years of Age* * This table includes all adverse events (whether considered by the investigator to be related or unrelated to drug) from these trials that occurred at a rate of 2% or greater in the group treated with XOPENEX HFA Inhalation Aerosol and more frequently than in the HFA-134a placebo inhaler group.
Body System
Preferred TermXOPENEX HFA Inhalation Aerosol
90 mcg
(n=403)Racemic Albuterol HFA
180 mcg
(n=179)Placebo
(n=166)Body as a Whole Pain 4.0 3.4 3.6 Central Nervous System Dizziness 2.7 0.6 1.8 Respiratory System Asthma 9.4 7.3 6.0 Pharyngitis 7.9 2.2 2.4 Rhinitis 7.4 2.2 3.0 Adverse events reported by less than 2% and at least 2 or more of the adolescent and adult patients receiving XOPENEX HFA Inhalation Aerosol and by a greater proportion than receiving HFA-134a placebo inhaler include cyst, flu syndrome, viral infection, constipation, gastroenteritis, myalgia, hypertension, epistaxis, lung disorder, acne, herpes simplex, conjunctivitis, ear pain, dysmenorrhea, hematuria, and vaginal moniliasis. There were no significant laboratory abnormalities observed in these studies.
Adverse event information concerning XOPENEX HFA Inhalation Aerosol in children is derived from a 4-week, randomized, double-blind trial of XOPENEX HFA Inhalation Aerosol, a marketed albuterol HFA inhaler, and an HFA-134a placebo inhaler in 150 children aged 4 to 11 years with asthma. Table 3 lists the adverse events reported for XOPENEX HFA Inhalation Aerosol in children at a rate of 2% or greater and more frequently than for placebo.
Table 3: Adverse Event Incidence (% of Patients) in a 4-Week Trial in Children Aged 4-11 Years* * This table includes all adverse events (whether considered by the investigator to be related or unrelated to drug) from the trial that occurred at a rate of 2% or greater in the group treated with XOPENEX HFA Inhalation Aerosol and more frequently than in the HFA-134a placebo inhaler group.
Body System
Preferred TermXOPENEX HFA Inhalation Aerosol
90 mcg
(n=76)Racemic Albuterol HFA
180 mcg
(n=39)Placebo
(n=35)Body as a Whole Accidental injury 9.2 10.3 5.7 Digestive System Vomiting 10.5 7.7 5.7 Respiratory System Bronchitis 2.6 0 0 Pharyngitis 6.6 12.8 5.7 The incidence of systemic beta-adrenergic adverse effects (e.g., tremor, nervousness) was low and comparable across all treatment groups, including placebo.
Postmarketing
In addition to the adverse events reported in clinical trials, the following adverse events have been observed in postapproval use of levalbuterol inhalation solution. These events have been chosen for inclusion due to their seriousness, their frequency of reporting, or their likely beta-mediated mechanism: angioedema, anaphylaxis, arrhythmias (including atrial fibrillation, supraventricular tachycardia, extrasystoles), asthma, chest pain, cough increased, dyspnea, nausea, nervousness, rash, tachycardia, tremor, urticaria. Because these events have been reported spontaneously from a population of unknown size, estimates of frequency cannot be made.
In addition, XOPENEX HFA Inhalation Aerosol, like other sympathomimetic agents, can cause adverse reactions such as hypertension, angina, vertigo, central nervous system stimulation, sleeplessness, headache, and drying or irritation of the oropharynx.
-
OVERDOSAGE
The expected symptoms with overdosage are those of excessive beta-adrenergic receptor stimulation and/or occurrence or exaggeration of any of the symptoms listed under ADVERSE REACTIONS, e.g., seizures, angina, hypertension or hypotension, tachycardia with rates up to 200 beats/minute, arrhythmias, nervousness, headache, tremor, dry mouth, palpitation, nausea, dizziness, fatigue, malaise, and sleeplessness. Hypokalemia also may occur. As with all sympathomimetic medications, cardiac arrest and even death may be associated with the abuse of XOPENEX HFA (levalbuterol tartrate) Inhalation Aerosol. Treatment consists of discontinuation of XOPENEX HFA Inhalation Aerosol together with appropriate symptomatic therapy. The judicious use of a cardioselective beta-receptor blocker may be considered, bearing in mind that such medication can produce bronchospasm. There is insufficient evidence to determine if dialysis is beneficial for overdosage of XOPENEX HFA Inhalation Aerosol.
Following intravenous administration in mice, the median lethal levalbuterol HCl dose was approximately 66 mg/kg (approximately 500 times the maximum recommended daily inhalation dose of levalbuterol tartrate for adults on a mg/m2 basis and approximately 230 times the maximum recommended daily inhalation dose of levalbuterol tartrate for pediatric patients on a mg/m2 basis). Following intravenous administration in rats, the median lethal levalbuterol HCl dose was approximately 60 mg/kg (approximately 900 times the maximum recommended daily inhalation dose of levalbuterol tartrate for adults on a mg/m2 basis and approximately 430 times the maximum recommended daily inhalation dose of levalbuterol tartrate for children on a mg/m2 basis). The inhalation median lethal dose has not been determined in animals. In dogs, inhaled doses of levalbuterol HCl up to 2.73 mg/kg (approximately 140 times the maximum recommended daily inhalation dose of levalbuterol tartrate for adults on a mg/m2 basis and approximately 65 times the maximum recommended daily inhalation dose of levalbuterol tartrate for children on a mg/m2 basis) were tolerated without animal deaths.
-
DOSAGE AND ADMINISTRATION
Adult and Pediatric Asthma: For treatment of acute episodes of bronchospasm or prevention of asthmatic symptoms, the usual dosage of XOPENEX HFA (levalbuterol tartrate) Inhalation Aerosol for adults and children 4 years of age and older is 2 inhalations (90 mcg) repeated every 4 to 6 hours; in some patients, 1 inhalation every 4 hours may be sufficient. More frequent administration or a larger number of inhalations is not routinely recommended. It is recommended to prime the inhaler before using for the first time and in cases where the inhaler has not been used for more than 3 days by releasing 4 test sprays into the air, away from the face.
If a previously effective dosage regimen fails to provide the usual response, this may be a marker of destabilization of asthma and requires reevaluation of the patient and the treatment regimen, giving special consideration to the possible need for anti-inflammatory treatment, e.g., corticosteroids.
Cleaning: To maintain proper use of this product, it is critical that the actuator be washed and dried thoroughly at least once a week. The inhaler may cease to deliver medication if not properly cleaned and dried thoroughly. See Information for Patients. Keeping the plastic actuator clean is very important to prevent medication build-up and blockage. If the actuator becomes blocked with drug, washing the actuator will remove the blockage.
-
HOW SUPPLIED
XOPENEX HFA (levalbuterol tartrate) Inhalation Aerosol is supplied as a pressurized aluminum canister in a box (NDC 54868-5689-0). The canister is labeled with a net weight of 15 g and contains 200 metered actuations (or inhalations), respectively. Each canister is supplied with a blue plastic actuator (or mouthpiece), a red mouthpiece cap, and patient's instructions.
SHAKE WELL BEFORE USING. Store between 20° and 25°C (68° and 77°F; see USP controlled room temperature). Protect from freezing temperatures and direct sunlight. Store inhaler with the actuator (or mouthpiece) down. Avoid spraying in eyes. Contents under pressure. Do not puncture or incinerate. Exposure to temperatures above 120°F may cause bursting. Keep out of reach of children.
The blue actuator supplied with XOPENEX HFA Inhalation Aerosol should not be used with any other product canisters. Actuators from other products should not be used with a XOPENEX HFA Inhalation Aerosol canister. The correct amount of medication in each actuation cannot be assured after 200 actuations from the 15 g canister or 80 actuations from the 8.4 g canister, even though the canister is not completely empty. The canister should be discarded when 200 actuations have been used from the 15 g canister or 80 actuations have been used from the 8.4 g canister.
XOPENEX HFA Inhalation Aerosol does not contain chlorofluorocarbons (CFCs) as the propellant.
Rx only.
Manufactured for:
Sepracor Inc.
Marlborough, MA 01752 USA
by 3M Drug Delivery Systems
Northridge, CA 91324-3213For customer service, call 1-888-394-7377.
To report adverse events, call 1-877-737-7226.
For medical information, call 1-800-739-0565.June 2009
900874R05
Additional barcode labeling by:
Physicians Total Care, Inc.
Tulsa, Oklahoma 74146
PHARMACIST — DETACH HERE AND GIVE INSTRUCTIONS TO PATIENT.
-----------------------------------------------------------------------------------------------------------
-
Patient's Instructions For Use
XOPENEX HFA® (levalbuterol tartrate) Inhalation Aerosol
Before using your XOPENEX HFA (levalbuterol tartrate) Inhalation Aerosol, read the complete instructions carefully.
ABOUT XOPENEX HFA INHALATION AEROSOL
Use only as directed by a doctor. Children should use XOPENEX HFA Inhalation Aerosol under adult supervision, as instructed by the patient's doctor.
XOPENEX HFA Inhalation Aerosol is a pressurized metered-dose inhaler that produces an aerosol for oral inhalation. XOPENEX HFA Inhalation Aerosol does not contain chlorofluorocarbons (CFCs).
The blue actuator (or mouthpiece) supplied with XOPENEX HFA Inhalation Aerosol should not be used with any other product canisters. Actuators from other products should not be used with a XOPENEX HFA Inhalation Aerosol canister.
HOW TO USE YOUR XOPENEX HFA INHALATION AEROSOL
- SHAKE THE INHALER WELL immediately before each use.
-
REMOVE THE CAP FROM THE ACTUATOR (OR MOUTHPIECE) (see Figure 1). Inspect the actuator for the presence of foreign objects and make sure that the canister is seated in the actuator before each use.
PRIMING: Priming at specified times is important for the proper delivery of your medication. SHAKE THE INHALER WELL; then prime XOPENEX HFA Inhalation Aerosol by releasing 4 test sprays into the air, away from your face, before using for the first time and when the inhaler has not been used for more than 3 days.
- BREATHE OUT FULLY THROUGH YOUR MOUTH, expelling as much air from your lungs as possible. Place the mouthpiece fully into your mouth, holding the inhaler in the mouthpiece-down position (see Figure 2) and closing your lips around it.
- WHILE BREATHING IN DEEPLY AND SLOWLY THROUGH YOUR MOUTH, FULLY DEPRESS THE TOP OF THE METAL CANISTER with your middle finger as shown in Figure 2. Immediately after the puff is delivered, release your finger from the canister and remove the inhaler from your mouth.
- HOLD YOUR BREATH FOR 10 SECONDS, IF POSSIBLE.
- If your doctor has prescribed more than a single inhalation/puff, wait 1 minute between inhalations. Then, SHAKE THE INHALER WELL and repeat steps 3 through 5.
- REPLACE THE CAP ON THE MOUTHPIECE AFTER EACH USE.
- CLEAN THE ACTUATOR OR MOUTHPIECE AT LEAST ONCE A WEEK. See CLEANING YOUR XOPENEX HFA INHALATION AEROSOL for cleaning instructions.
- DISCARD THE CANISTER AFTER YOU HAVE USED EITHER 200 INHALATIONS FROM THE 15 g CANISTER OR 80 INHALATIONS FROM THE 8.4 g CANISTER. The correct amount of medicine in each inhalation cannot be assured after 200 sprays from the 15 g canister or 80 sprays from the 8.4 g canister, even though the canister is not completely empty. Never immerse the canister in water to determine how full the canister is (“float test”). Before you reach either 200 sprays or 80 sprays, you should consult your doctor to determine whether a refill is needed. Just as you should not take extra doses without consulting your doctor, you also should not stop using XOPENEX HFA Inhalation Aerosol without consulting your doctor.
CLEANING YOUR XOPENEX HFA INHALATION AEROSOL
KEEPING THE BLUE PLASTIC ACTUATOR (OR MOUTHPIECE) CLEAN IS VERY IMPORTANT TO PREVENT MEDICINE BLOCKAGE. THE ACTUATOR SHOULD BE WASHED, SHAKEN TO REMOVE EXCESS WATER, AND AIR-DRIED THOROUGHLY AT LEAST ONCE A WEEK. THE INHALER MAY STOP WORKING IF NOT PROPERLY CLEANED.
ROUTINE CLEANING INSTRUCTIONS:
- Step 1. To clean the blue plastic actuator (or mouthpiece), remove the canister and red mouthpiece cap.
-
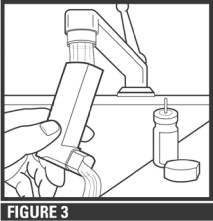
Step 2. Wash the actuator through the top and bottom with warm running water for 30 seconds at least once a week (see Figure 3).
Do not clean the metal canister or allow the metal canister to become wet. Never immerse the metal canister in water.
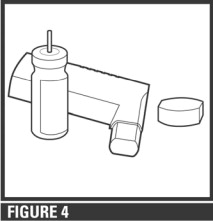
- Step 3. To dry, shake off excess water and let the actuator air-dry thoroughly, such as overnight (see Figure 4).
- Step 4. When the actuator is dry, replace the canister and the mouthpiece cap; make sure the canister is fully and firmly inserted into the actuator. Blockage from medicine build-up is more likely to occur if the actuator is not allowed to air-dry thoroughly.
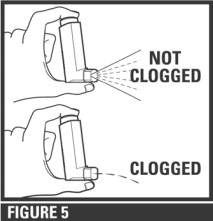
IF YOUR ACTUATOR BECOMES BLOCKED (little or no medicine coming out of the mouthpiece, see Figure 5), wash your actuator as described in Steps 1 and 2 and air-dry thoroughly as described in Step 3.
IF YOU NEED TO USE YOUR INHALER BEFORE THE PLASTIC ACTUATOR IS COMPLETELY DRY, SHAKE EXCESS WATER off the actuator, replace the canister, shake well, and test-spray twice into the air, away from your face, to remove most of the water remaining in the actuator. Then take your dose as prescribed. After such use, rewash the actuator and air-dry it thoroughly as described in Steps 1 through 3.
ADDITIONAL INFORMATION ABOUT XOPENEX HFA INHALATION AEROSOL
DOSAGE: Use only as directed by your doctor.
WARNINGS: The action of XOPENEX HFA Inhalation Aerosol should last for 4 to 6 hours. XOPENEX HFA Inhalation Aerosol should not be used more frequently than recommended. Do not increase the dose or frequency of doses of XOPENEX HFA Inhalation Aerosol without consulting your physician. If you find that treatment with XOPENEX HFA Inhalation Aerosol becomes less effective for symptomatic relief, your symptoms become worse, and/or you need to use the product more frequently than usual, you should seek medical attention immediately. While you are using XOPENEX HFA Inhalation Aerosol, other inhaled drugs and asthma medication should be taken only as directed by your physician.
Common adverse effects include palpitations, chest pain, rapid heart rate, tremor, and nervousness. If you are pregnant or nursing, contact your physician about the use of XOPENEX HFA Inhalation Aerosol. Effective and safe use of XOPENEX HFA Inhalation Aerosol includes an understanding of the way that it should be administered. In general, the technique for administering XOPENEX HFA Inhalation Aerosol to children is similar to that for adults. Children should use XOPENEX HFA Inhalation Aerosol under adult supervision, as instructed by the patient's physician.
Storage: Store canister between 20° and 25°C (68° and 77°F). Protect from freezing temperatures and direct sunlight. Store inhaler with the actuator (or mouthpiece) down. Contents under pressure. Do not puncture or incinerate. Exposure to temperatures above 120°F may cause bursting. Avoid spraying in eyes. Keep out of reach of children.
CFC-Free: XOPENEX HFA Inhalation Aerosol does not contain chlorofluorocarbons (CFCs). Instead, the inhaler contains a hydrofluoroalkane (HFA-134a) as the propellant.
Manufactured for:
Sepracor Inc.
Marlborough, MA 01752 USA
by 3M Drug Delivery Systems
Northridge, CA 91324-3213For customer service, call 1-888-394-7377.
To report adverse events, call 1-877-737-7226.
For medical information, call 1-800-739-0565.June 2009
900874R05
- TRADE CARTON - PRINCIPAL DISPLAY PANEL - 45 MCG 200 ACTUATIONS
-
INGREDIENTS AND APPEARANCE
XOPENEX HFA
levalbuterol tartrate aerosol, meteredProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:54868-5689(NDC:63402-510) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength levalbuterol tartrate (UNII: ADS4I3E22M) (levalbuterol - UNII:EDN2NBH5SS) levalbuterol 45 ug Inactive Ingredients Ingredient Name Strength norflurane (UNII: DH9E53K1Y8) Alcohol (UNII: 3K9958V90M) Oleic Acid (UNII: 2UMI9U37CP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54868-5689-0 200 in 1 INHALER Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021730 10/01/2007 Labeler - Physicians Total Care, Inc. (194123980) Establishment Name Address ID/FEI Business Operations Physicians Total Care, Inc. 194123980 relabel