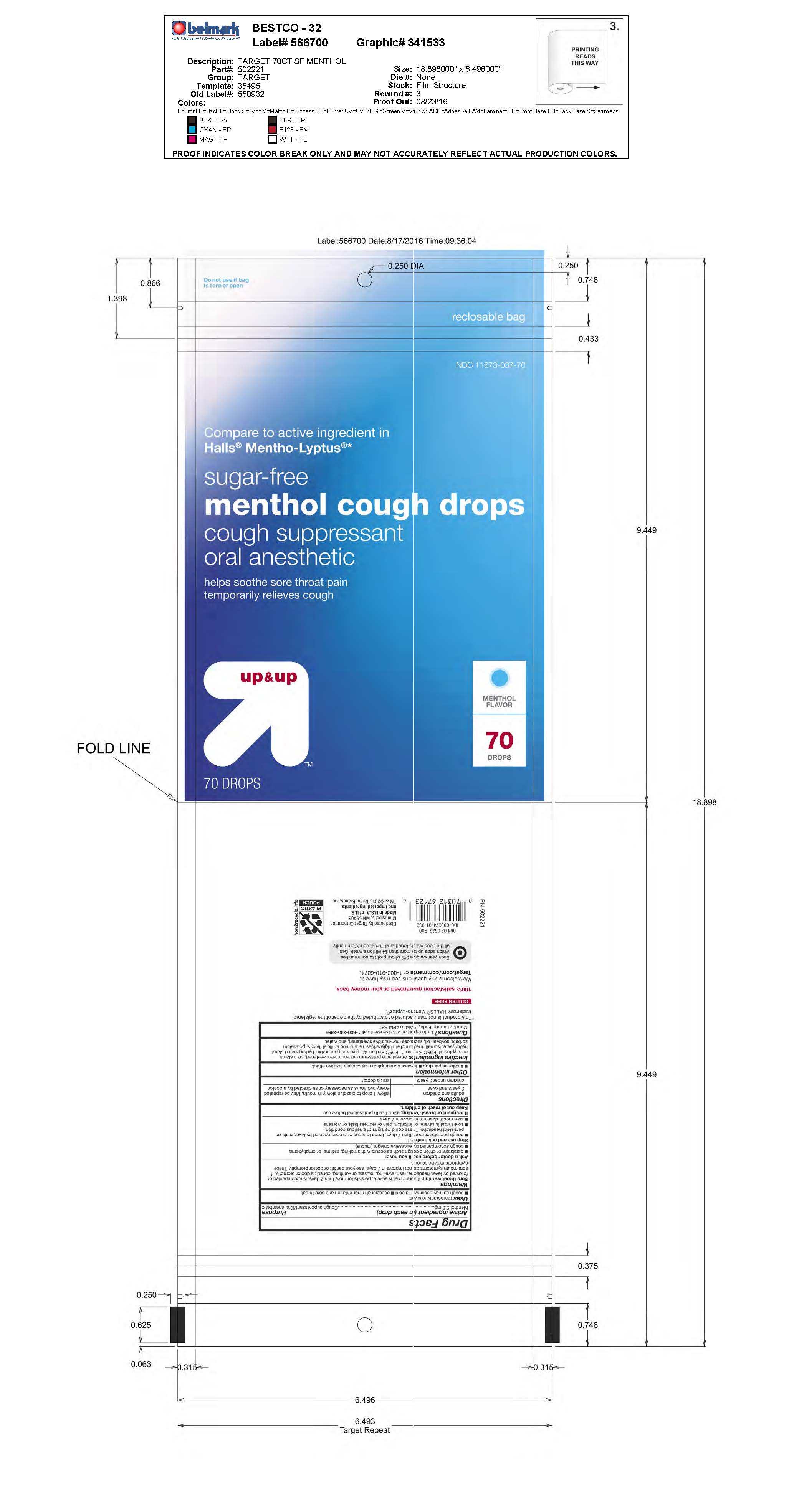
SUGAR FREE MENTHOL COUGH DROPS- menthol lozenge
Target Corporation
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Menthol 5.8 mg
Uses temporarily relieves:
cough as may occur with a cold
occasional minor irritation and sore throat
Warnings
Sore throat warning: If sore throat is severe, persists for more than 2 days, is accompanied or followed by fever, headache, rash, swelling, nausea, or vomiting, consult a doctor promptly. If sore mouth symptoms do not improve in 7 days, see your dentist or doctor promptly. These symptoms may be serious.
Ask a doctor before use if you have:
persistent or chronic cough such as occurs with smoking, asthma, or emphysema
cough accompained by excessive phlegm (mucus)
Stop use and consult doctor if
cough persists for more than 7 days, tends to recur, or is accompanied by fever, rash, or persistent headache. These could be signs of a serious condition.
sore throat is severe, or irritation, pain or redness lasts or worsens
sore mouth does not improve in 7 days
Directions
adults and children 5 years and over - dissolve 1 lozenge slowly in mouth. Repeat every 2 hours as needed or as directed by a doctor.
children under 5 years - ask a doctor
Inactive ingredients: acesulfame potassium (non-nutritive sweetener), corn starch, eucalyptus oil, FD&C Blue no. 1, FD&C Red no. 40, glycerin, gum arabic, hydrogenated starch hydrolysate, isomalt, medium chain triglycerides, natural and artificial flavors, potassium sorbate, soybean oil, sucralose (non-nutritive sweetener), and water.
| SUGAR FREE MENTHOL COUGH DROPS
menthol lozenge |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
 |
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Target Corporation (006961700) |
| Registrant - Bestco Inc. (002149136) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Bestco Inc. | 002149136 | manufacture(11673-037) | |