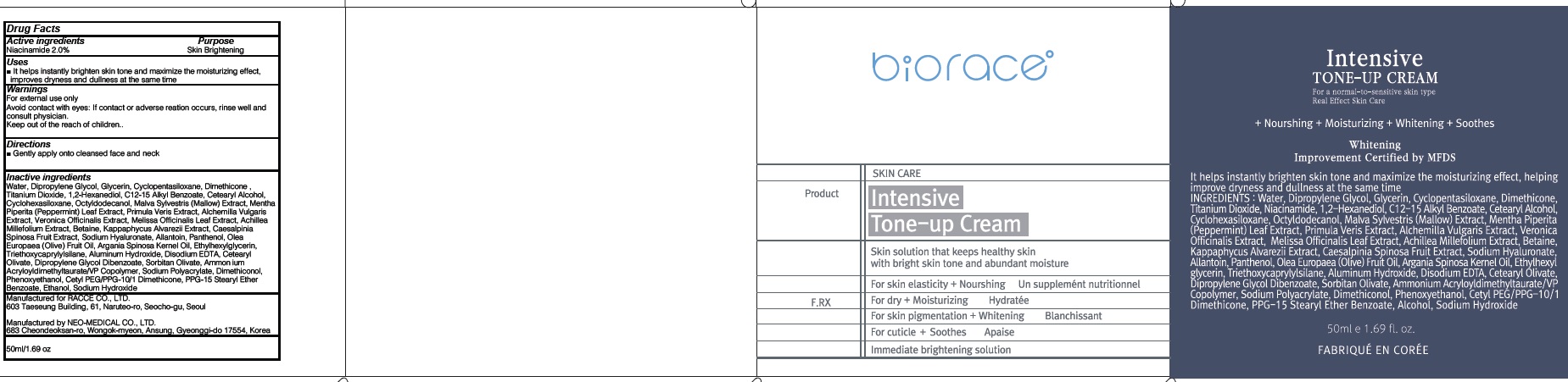
INTENSIVE TONE UP- niacinamide cream
Racce Co., Ltd.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
INACTIVE INGREDIENT
Inactive ingredients:
Water, Dipropylene Glycol, Glycerin, Cyclopentasiloxane, Dimethicone, Titanium Dioxide, 1,2-Hexanediol, C12-15 Alkyl Benzoate, Cetearyl Alcohol, Cyclohexasiloxane, Octyldodecanol, Malva Sylvestris (Mallow) Extract, Mentha Piperita (Peppermint) Leaf Extract, Primula Veris Extract, Alchemilla Vulgaris Extract, Veronica Officinalis Extract, Melissa Officinalis Leaf Extract, Achillea Millefolium Extract, Betaine, Kappaphycus Alvarezii Extract, Caesalpinia Spinosa Fruit Extract, Sodium Hyaluronate, Allantoin, Panthenol, Olea Europaea (Olive) Fruit Oil, Argania Spinosa Kernel Oil, Ethylhexylglycerin, Triethoxycaprylylsilane, Aluminum Hydroxide, Disodium EDTA, Cetearyl Olivate, Dipropylene Glycol Dibenzoate, Sorbitan Olivate, Ammonium Acryloyldimethyltaurate/VP Copolymer, Sodium Polyacrylate, Dimethiconol, Phenoxyethanol, Cetyl PEG/PPG-10/1 Dimethicone, PPG-15 Stearyl Ether Benzoate, Ethanol, Sodium Hydroxide
WARNINGS
Warnings:
For external use only
Avoid contact with eyes: If contact or adverse reation occurs, rinse well and consult physician.
Keep out of the reach of children.
| INTENSIVE TONE UP
niacinamide cream |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Racce Co., Ltd. (694890309) |
| Registrant - Racce Co., Ltd. (694890309) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| NEO-MEDICAL CO.,LTD | 689755531 | manufacture(72092-080) | |