HAND KLEEN FOAMING ANTIBACTERIAL HAND- triclosan liquid
Auto-Chlor System, LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
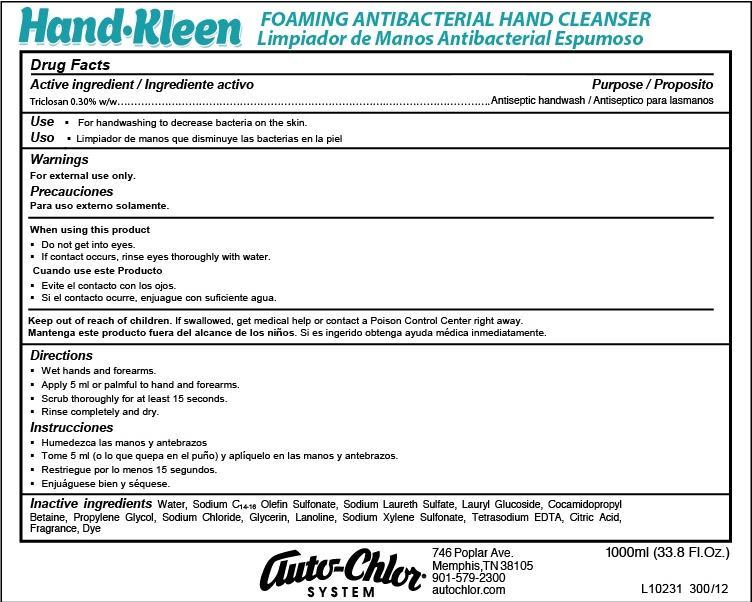
Drug Facts
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
For external use only.
When using this product
Do not get into eyes.
If contact occurs, rinse thoroughly with water.
| HAND KLEEN FOAMING ANTIBACTERIAL HAND
triclosan liquid |
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
| Labeler - Auto-Chlor System, LLC (965194330) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Auto-Chlor System, LLC | 965194330 | manufacture(68604-231) | |
Revised: 9/2017
Document Id: 5a443573-b45a-420e-e053-2a91aa0a1696
Set id: 3117562b-3e77-40fb-9dee-b38ecc237106
Version: 2
Effective Time: 20170928
Auto-Chlor System, LLC