TIVICAY- dolutegravir sodium tablet, film coated
REMEDYREPACK INC.
----------
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use TIVICAY safely and effectively. See full prescribing information for TIVICAY.
TIVICAY (dolutegravir) tablets, for oral use Initial U.S. Approval: 2013 RECENT MAJOR CHANGES
INDICATIONS AND USAGETIVICAY is a human immunodeficiency virus type 1 (HIV-1) integrase strand transfer inhibitor (INSTI) indicated in combination with:
DOSAGE AND ADMINISTRATIONMay be taken without regard to food. ( 2)
a Rilpivirine dose is 25 mg once daily for those switching to dolutegravir plus rilpivirine. b Alternative combinations that do not include metabolic inducers should be considered where possible. Pediatric Patients: (Treatment-naïve or treatment-experienced INSTI-naïve patients weighing at least 30 kg). ( 2.2) DOSAGE FORMS AND STRENGTHSTablets: 10 mg, 25 mg, and 50 mg ( 3) CONTRAINDICATIONSWARNINGS AND PRECAUTIONS
ADVERSE REACTIONSThe most common adverse reactions of moderate to severe intensity and incidence at least 2% (in those receiving TIVICAY in any one adult trial) are insomnia, fatigue, and headache. ( 6.1) To report SUSPECTED ADVERSE REACTIONS, contact ViiV Healthcare at 1-877-844-8872 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. DRUG INTERACTIONS
USE IN SPECIFIC POPULATIONSSee 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling. Revised: 6/2018 |
FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
TIVICAY is indicated in combination with:TIVICAY is indicated in combination with:
- other antiretroviral agents for the treatment of human immunodeficiency virus type 1 (HIV-1) infection in adults and pediatric patients weighing at least 30 kg [see Microbiology ( 12.4)] .
- rilpivirine as a complete regimen for the treatment of HIV-1 infection in adults to replace the current antiretroviral regimen in those who are virologically suppressed (HIV-1 RNA less than 50 copies per mL) on a stable antiretroviral regimen for at least 6 months with no history of treatment failure or known substitutions associated with resistance to either antiretroviral agent.rilpivirine as a complete regimen for the treatment of HIV-1 infection in adults to replace the current antiretroviral regimen in those who are virologically suppressed (HIV-1 RNA less than 50 copies per mL) on a stable antiretroviral regimen for at least 6 months with no history of treatment failure or known substitutions associated with resistance to either antiretroviral agent.
2 DOSAGE AND ADMINISTRATION
2.1 Adults
TIVICAY tablets may be taken with or without food.TIVICAY tablets may be taken with or without food.
|
Population |
Recommended Dose |
|
Treatment-naïve or treatment-experienced INSTI-naïve or virologically suppressed (HIV-1 RNA <50 copies per mL) adults switching to dolutegravir plus rilpivirine a |
50 mg once daily |
|
Treatment-naïve or treatment-experienced INSTI-naïve when coadministered with certain UGT1A or CYP3A inducers Treatment-naïve or treatment-experienced INSTI-naïve when coadministered with certain UGT1A or CYP3A inducers [see Drug Interactions ( 7.3)] |
50 mg twice daily50 mg twice daily |
|
INSTI-experienced with certain INSTI-associated resistance substitutions or clinically suspected INSTI resistance INSTI-experienced with certain INSTI-associated resistance substitutions or clinically suspected INSTI resistance b [see Microbiology ( 12.4)] |
50 mg twice daily50 mg twice daily |
Rilpivirine dose is 25 mg once daily for those switching to dolutegravir plus rilpivirine. a Rilpivirine dose is 25 mg once daily for those switching to dolutegravir plus rilpivirine.
Alternative combinations that do not include metabolic inducers should be considered where possible . b Alternative combinations that do not include metabolic inducers should be considered where possible [see Drug Interactions ( 7)] .
2.2 Pediatric Patients
TIVICAY tablets may be taken with or without food.
Treatment-Naïve or Treatment-Experienced INSTI-Naïve
The recommended dose of TIVICAY in pediatric patients weighing at least 30 kg is provided in Table 2.
|
Body Weight (kg) |
Daily Dose a (Number of Tablets per Dose when Different Strength(s) are Required) |
|
30 to less than 40 |
35 mg once daily
|
|
40 or greater |
50 mg once daily |
a If certain UGT1A or CYP3A inducers are coadministered, then increase the weight-based dose of TIVICAY to twice daily [see Drug Interactions ( 7.3) for relevant inducers] .
Safety and efficacy of TIVICAY have not been established in pediatric patients who are INSTI-experienced with documented or clinically suspected resistance to other INSTIs (raltegravir, elvitegravir).
3 DOSAGE FORMS AND STRENGTHS
Tablets:
10 mg: Each tablet contains 10 mg of dolutegravir (as dolutegravir sodium). Tablets are white, round, film-coated, biconvex tablets debossed with “SV 572” on one side and “10” on the other side.
25 mg: Each tablet contains 25 mg of dolutegravir (as dolutegravir sodium). Tablets are pale yellow, round, film-coated, biconvex tablets debossed with “SV 572” on one side and “25” on the other side.
50 mg: Each tablet contains 50 mg of dolutegravir (as dolutegravir sodium). Tablets are yellow, round, film-coated, biconvex tablets debossed with “SV 572” on one side and “50” on the other side.
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions
Hypersensitivity reactions have been reported and were characterized by rash, constitutional findings, and sometimes organ dysfunction, including liver injury. The events were reported in less than 1% of subjects receiving TIVICAY in Phase 3 clinical trials. Discontinue TIVICAY and other suspect agents immediately if signs or symptoms of hypersensitivity reactions develop (including, but not limited to, severe rash or rash accompanied by fever, general malaise, fatigue, muscle or joint aches, blisters or peeling of the skin, oral blisters or lesions, conjunctivitis, facial edema, hepatitis, eosinophilia, angioedema, difficulty breathing). Clinical status, including liver aminotransferases, should be monitored and appropriate therapy initiated. Delay in stopping treatment with TIVICAY or other suspect agents after the onset of hypersensitivity may result in a life-threatening reaction. TIVICAY is contraindicated in patients who have experienced a previous hypersensitivity reaction to dolutegravir.
5.2 Hepatotoxicity
Hepatic adverse events have been reported in patients receiving a dolutegravir-containing regimen. Patients with underlying hepatitis B or C may be at increased risk for worsening or development of transaminase elevations with use of TIVICAY [see Adverse Reactions ( 6.1)] . In some cases, the elevations in transaminases were consistent with immune reconstitution syndrome or hepatitis B reactivation particularly in the setting where anti-hepatitis therapy was withdrawn. Cases of hepatic toxicity, including elevated serum liver biochemistries, hepatitis, and acute liver failure have been reported in patients receiving a dolutegravir-containing regimen without pre-existing hepatic disease or other identifiable risk factors. Drug-induced liver injury leading to liver transplant has been reported with TRIUMEQ (abacavir, dolutegravir, and lamivudine). Monitoring for hepatotoxicity is recommended.
5.3 Risk of Adverse Reactions or Loss of Virologic Response Due to Drug Interactions
The concomitant use of TIVICAY and other drugs may result in known or potentially significant drug interactions, some of which may lead to [see Contraindications ( 4), Drug Interactions ( 7.3)]:
- Loss of therapeutic effect of TIVICAY and possible development of resistance.Loss of therapeutic effect of TIVICAY and possible development of resistance.
- Possible clinically significant adverse reactions from greater exposures of concomitant drugs.Possible clinically significant adverse reactions from greater exposures of concomitant drugs.
For concomitant drugs for which the interaction can be mitigated, please see Table 6 for steps to prevent or manage these possible and known significant drug interactions, including dosing recommendations. Consider the potential for drug interactions prior to and during therapy with TIVICAY; review concomitant medications during therapy with TIVICAY; and monitor for the adverse reactions associated with the concomitant drugs.
5.4 Immune Reconstitution Syndrome
Immune reconstitution syndrome has been reported in patients treated with combination antiretroviral therapy, including TIVICAY. During the initial phase of combination antiretroviral treatment, patients whose immune systems respond may develop an inflammatory response to indolent or residual opportunistic infections (such as Mycobacterium avium infection, cytomegalovirus, Pneumocystis jirovecii pneumonia [PCP], or tuberculosis), which may necessitate further evaluation and treatment.
Autoimmune disorders (such as Graves’ disease, polymyositis, and Guillain-Barré syndrome) have also been reported to occur in the setting of immune reconstitution; however, the time to onset is more variable and can occur many months after initiation of treatment.
6 ADVERSE REACTIONS
The following serious adverse drug reactions are discussed in other sections of the labeling:
- Hypersensitivity reactions [see Warnings and Precautions ( 5.1)] .
- Hepatotoxicity [see Warnings and Precautions ( 5.2)].
- Immune Reconstitution Syndrome [see Warnings and Precautions ( 5.4)] .
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared with rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Clinical Trials Experience in Adult Subjects
Treatment-Naïve Subjects: The safety assessment of TIVICAY in HIV‑1‑infected treatment-naïve subjects is based on the analyses of data from 2 international, multicenter, double-blind trials, SPRING-2 (ING113086) and SINGLE (ING114467) and data from the international, multicenter, open-label FLAMINGO (ING114915) trial.
In SPRING-2, 822 subjects were randomized and received at least 1 dose of either TIVICAY 50 mg once daily or raltegravir 400 mg twice daily, both in combination with fixed-dose dual nucleoside reverse transcriptase inhibitor (NRTI) treatment (either abacavir sulfate and lamivudine [EPZICOM] or emtricitabine/tenofovir [TRUVADA]). There were 808 subjects included in the efficacy and safety analyses. Through 96 weeks, the rate of adverse events leading to discontinuation was 2% in both treatment arms.
In SINGLE, 833 subjects were randomized and received at least 1 dose of either TIVICAY 50 mg with fixed-dose abacavir sulfate and lamivudine (EPZICOM) once daily or fixed-dose efavirenz/emtricitabine/tenofovir (ATRIPLA) once daily (study treatment was blinded through Week 96 and open-label from Week 96 through Week 144). Through 144 weeks, the rates of adverse events leading to discontinuation were 4% in subjects receiving TIVICAY 50 mg once daily + EPZICOM and 14% in subjects receiving ATRIPLA once daily.
Treatment-emergent adverse reactions (ARs) of moderate to severe intensity observed in at least 2% of subjects in either treatment arm in SPRING-2 and SINGLE trials are provided in Table 3. Side-by-side tabulation is to simplify presentation; direct comparisons across trials should not be made due to differing trial designs.
|
System Organ Class/ Preferred Term |
SPRING-2 |
SINGLE |
||
|
TIVICAY 50 mg Once Daily + 2 NRTIs
|
Raltegravir
400 mg Twice Daily + 2 NRTIs
|
TIVICAY 50 mg + EPZICOM Once Daily
|
ATRIPLA Once Daily
|
|
|
Psychiatric | ||||
|
Insomnia |
<1% |
<1% |
3% |
3% |
|
Depression |
<1% |
<1% |
1% |
2% |
|
Abnormal dreams |
<1% |
<1% |
<1% |
2% |
|
Nervous System | ||||
|
Dizziness |
<1% |
<1% |
<1% |
5% |
|
Headache |
<1% |
<1% |
2% |
2% |
|
Gastrointestinal | ||||
|
Nausea |
1% |
1% |
<1% |
3% |
|
Diarrhea |
<1% |
<1% |
<1% |
2% |
|
Skin and Subcutaneous Tissue | ||||
|
Rash a |
0 |
<1% |
<1% |
6% |
|
General Disorders | ||||
|
Fatigue |
<1% |
<1% |
2% |
2% |
|
Ear and Labyrinth | ||||
|
Vertigo |
0 |
<1% |
0 |
2% |
a Includes pooled terms: rash, rash generalized, rash macular, rash maculo-papular, rash pruritic, and drug eruption.
In addition, Grade 1 insomnia was reported by 1% and less than 1% of subjects receiving TIVICAY and raltegravir, respectively, in SPRING-2; whereas in SINGLE the rates were 7% and 4% for TIVICAY and ATRIPLA, respectively. These events were not treatment limiting.
In a multicenter, open-label trial (FLAMINGO), 243 subjects received TIVICAY 50 mg once daily versus 242 subjects who received darunavir 800 mg/ritonavir 100 mg once daily, both in combination with investigator-selected NRTI background regimen (either EPZICOM or TRUVADA). There were 484 subjects included in the efficacy and safety analyses. Through 96 weeks, the rates of adverse events leading to discontinuation were 3% in subjects receiving TIVICAY and 6% in subjects receiving darunavir/ritonavir. The ARs observed in FLAMINGO were generally consistent with those seen in SPRING-2 and SINGLE.
Treatment-Experienced, Integrase Strand Transfer Inhibitor-Naïve Subjects: In an international, multicenter, double-blind trial (ING111762, SAILING), 719 HIV‑1‑infected, antiretroviral treatment-experienced adults were randomized and received either TIVICAY 50 mg once daily or raltegravir 400 mg twice daily with investigator-selected background regimen consisting of up to 2 agents, including at least one fully active agent. At 48 weeks, the rates of adverse events leading to discontinuation were 3% in subjects receiving TIVICAY 50 mg once daily + background regimen and 4% in subjects receiving raltegravir 400 mg twice daily + background regimen.
The only treatment-emergent AR of moderate to severe intensity with at least 2% frequency in either treatment group was diarrhea, 2% (6 of 354) in subjects receiving TIVICAY 50 mg once daily + background regimen and 1% (5 of 361) in subjects receiving raltegravir 400 mg twice daily + background regimen.
Treatment-Experienced, Integrase Strand Transfer Inhibitor-Experienced Subjects: In a multicenter, open-label, single-arm trial (ING112574, VIKING-3), 183 HIV‑1‑infected, antiretroviral treatment-experienced adults with virological failure and current or historical evidence of raltegravir and/or elvitegravir resistance received TIVICAY 50 mg twice daily with the current failing background regimen for 7 days and with optimized background therapy from Day 8. The rate of adverse events leading to discontinuation was 4% of subjects at Week 48.
Treatment-emergent ARs in VIKING-3 were generally similar compared with observations with the 50-mg once-daily dose in adult Phase 3 trials.
Virologically Suppressed Subjects: The ARs observed for TIVICAY plus rilpivirine in the Week 48 analysis of pooled data from two identical, international, multicenter, open-label trials (SWORD-1 and SWORD-2) of 513 HIV–1-infected, virologically suppressed subjects switching from their current antiretroviral regimen to dolutegravir plus rilpivirine, were consistent with the AR profiles and severities for the individual components when administered with other antiretroviral agents. There were no ARs (Grades 2 to 4) with an incidence of at least 2% in either treatment arm. The rates of adverse events leading to discontinuation were 4% in subjects receiving TIVICAY plus rilpivirine once daily and less than 1% in subjects who remained on their current antiretroviral regimen.
Less Common Adverse Reactions Observed in Treatment-Naïve and Treatment-Experienced Trials: The following ARs occurred in less than 2% of treatment-naïve or treatment-experienced subjects receiving TIVICAY in a combination regimen in any one trial. These events have been included because of their seriousness and assessment of potential causal relationship.
Gastrointestinal Disorders: Abdominal pain, abdominal discomfort, flatulence, upper abdominal pain, vomiting.
Hepatobiliary Disorders: Hepatitis.
Musculoskeletal Disorders: Myositis.
Psychiatric Disorders: Suicidal ideation, attempt, behavior, or completion. These events were observed primarily in subjects with a pre-existing history of depression or other psychiatric illness.
Renal and Urinary Disorders: Renal impairment.
Skin and Subcutaneous Tissue Disorders: Pruritus.
Laboratory Abnormalities:
Treatment-Naïve Subjects: Selected laboratory abnormalities (Grades 2 to 4) with a worsening grade from baseline and representing the worst-grade toxicity in at least 2% of subjects are presented in Table 4. The mean change from baseline observed for selected lipid values is presented in Table 5. Side-by-side tabulation is to simplify presentation; direct comparisons across trials should not be made due to differing trial designs.
|
Laboratory Parameter Preferred Term |
SPRING-2 |
SINGLE |
||
|
TIVICAY 50 mg Once Daily + 2 NRTIs (n = 403) |
Raltegravir 400 mg Twice Daily + 2 NRTIs (n = 405) |
TIVICAY 50 mg + EPZICOM Once Daily (n = 414) |
ATRIPLA Once Daily (n = 419) |
|
|
ALT | ||||
|
Grade 2 (>2.5-5.0 x ULN) |
4% |
4% |
3% |
5% |
|
Grade 3 to 4 (>5.0 x ULN) |
2% |
2% |
1% |
<1% |
|
AST | ||||
|
Grade 2 (>2.5-5.0 x ULN) |
5% |
3% |
3% |
4% |
|
Grade 3 to 4 (>5.0 x ULN) |
3% |
2% |
1% |
3% |
|
Total Bilirubin | ||||
|
Grade 2 (1.6-2.5 x ULN) |
3% |
2% |
<1% |
<1% |
|
Grade 3 to 4 (>2.5 x ULN) |
<1% |
<1% |
<1% |
<1% |
|
Creatine kinase | ||||
|
Grade 2 (6.0-9.9 x ULN) |
2% |
5% |
5% |
3% |
|
Grade 3 to 4 (≥10.0 x ULN) |
7% |
4% |
7% |
8% |
|
Hyperglycemia | ||||
|
Grade 2 (126-250 mg/dL) |
6% |
6% |
9% |
6% |
|
Grade 3 (>250 mg/dL) |
<1% |
2% |
2% |
<1% |
|
Lipase | ||||
|
Grade 2 (>1.5-3.0 x ULN) |
7% |
7% |
11% |
11% |
|
Grade 3 to 4 (>3.0 x ULN) |
2% |
5% |
5% |
4% |
|
Total neutrophils | ||||
|
Grade 2 (0.75-0.99 x 10 9) |
4% |
3% |
4% |
5% |
|
Grade 3 to 4 (<0.75 x 10 9) |
2% |
2% |
3% |
3% |
ULN = Upper limit of normal.
|
Laboratory Parameter Preferred Term |
SPRING-2 |
SINGLE |
||
|
TIVICAY 50 mg Once Daily + 2 NRTIs (n = 403) |
Raltegravir 400 mg Twice Daily + 2 NRTIs (n = 405) |
TIVICAY 50 mg + EPZICOM Once Daily (n = 414) |
ATRIPLA Once Daily (n = 419) |
|
|
Cholesterol (mg/dL) |
8.1 |
10.1 |
24.0 |
26.7 |
|
HDL cholesterol (mg/dL) |
2.0 |
2.3 |
5.4 |
7.2 |
|
LDL cholesterol (mg/dL) |
5.1 |
6.1 |
16.0 |
14.6 |
|
Triglycerides (mg/dL) |
6.7 |
6.6 |
13.6 |
31.9 |
a Subjects on lipid-lowering agents at baseline were excluded from these analyses (19 subjects in each arm in SPRING-2, and in SINGLE: TIVICAY + EPZICOM n = 30 and ATRIPLA n = 27). Ninety-four subjects initiated a lipid-lowering agent post-baseline; their last fasted on-treatment values (prior to starting the agent) were used regardless if they discontinued the agent (SPRING-2: TIVICAY n = 9, raltegravir n = 13; SINGLE: TIVICAY + EPZICOM n = 36 and ATRIPLA: n = 36).
Laboratory abnormalities observed in the FLAMINGO trial were generally consistent with observations in SPRING-2 and SINGLE.
Treatment-Experienced, Integrase Strand Transfer Inhibitor-Naïve Subjects: Laboratory abnormalities observed in SAILING were generally similar compared with observations seen in the treatment-naïve (SPRING-2 and SINGLE) trials.
Treatment-Experienced, Integrase Strand Transfer Inhibitor-Experienced Subjects: The most common treatment-emergent laboratory abnormalities (greater than 5% for Grades 2 to 4 combined) observed in VIKING-3 at Week 48 were elevated ALT (9%), AST (8%), cholesterol (10%), creatine kinase (6%), hyperglycemia (14%), and lipase (10%). Two percent (4 of 183) of subjects had a Grade 3 to 4 treatment-emergent hematology laboratory abnormality, with neutropenia (2% [3 of 183]) being the most frequently reported.
Virologically Suppressed Adults: Laboratory abnormalities observed in SWORD-1 and SWORD-2 were generally similar compared with observations seen in the other Phase 3 trials.
Hepatitis B and/or Hepatitis C Virus Co-infection: In Phase 3 trials, subjects with hepatitis B and/or C virus co-infection were permitted to enroll provided that baseline liver chemistry tests did not exceed 5 times the upper limit of normal. Overall, the safety profile in subjects with hepatitis B and/or C virus co-infection was similar to that observed in subjects without hepatitis B or C co-infection, although the rates of AST and ALT abnormalities were higher in the subgroup with hepatitis B and/or C virus co-infection for all treatment groups. Grades 2 to 4 ALT abnormalities in hepatitis B and/or C co-infected compared with HIV mono-infected subjects receiving TIVICAY were observed in 18% vs. 3% with the 50-mg once-daily dose and 13% vs. 8% with the 50-mg twice-daily dose. Liver chemistry elevations consistent with immune reconstitution syndrome were observed in some subjects with hepatitis B and/or C at the start of therapy with TIVICAY, particularly in the setting where anti-hepatitis therapy was withdrawn [see Warnings and Precautions ( 5.2)] .
Changes in Serum Creatinine: Dolutegravir has been shown to increase serum creatinine due to inhibition of tubular secretion of creatinine without affecting renal glomerular function [see Clinical Pharmacology ( 12.2)] . Increases in serum creatinine occurred within the first 4 weeks of treatment and remained stable through 96 weeks. In treatment-naïve subjects, a mean change from baseline of 0.15 mg per dL (range: -0.32 mg per dL to 0.65 mg per dL) was observed after 96 weeks of treatment. Creatinine increases were comparable by background NRTIs and were similar in treatment-experienced subjects.
Clinical Trials Experience in Pediatric Subjects
IMPAACT P1093 is an ongoing multicenter, open-label, non-comparative trial of approximately 160 HIV‑1‑infected pediatric subjects aged 4 weeks to less than 18 years, of which 46 treatment-experienced, INSTI-naïve subjects aged 6 to less than 18 years have been enrolled [see Use in Specific Populations ( 8.4), Clinical Studies ( 14.2)].
The adverse reaction profile was similar to that for adults. Grade 2 ARs reported by more than one subject were decreased neutrophil count (n = 3) and diarrhea (n = 2). There were no Grade 3 or 4 drug-related ARs reported. No ARs led to discontinuation.
The Grade 3 or 4 laboratory abnormalities reported in more than one subject were elevated total bilirubin (n = 3) and decreased neutrophil count (n = 2). The changes in mean serum creatinine were similar to those observed in adults.
6.2 Postmarketing Experience
In addition to adverse reactions reported from clinical trials, the following adverse reactions have been identified during postmarketing use. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Hepatobiliary Disorders
Acute liver failure, hepatotoxicity.
Musculoskeletal
Arthralgia, myalgia.
Psychiatric
Anxiety
7 DRUG INTERACTIONS
7.1 Effect of Dolutegravir on the Pharmacokinetics of Other Agents
In vitro, dolutegravir inhibited the renal organic cation transporters, OCT2 (IC 50 = 1.93 microM) and multidrug and toxin extrusion transporter (MATE) 1 (IC 50 = 6.34 microM). In vivo, dolutegravir inhibits tubular secretion of creatinine by inhibiting OCT2 and potentially MATE1. Dolutegravir may increase plasma concentrations of drugs eliminated via OCT2 or MATE1 (dofetilide and metformin, Table 6) [see Contraindications ( 4), Drug Interactions ( 7.3)] .
In vitro, dolutegravir inhibited the basolateral renal transporters, organic anion transporter (OAT) 1 (IC 50 = 2.12 microM) and OAT3 (IC 50 = 1.97 microM). However, in vivo, dolutegravir did not alter the plasma concentrations of tenofovir or para-amino hippurate, substrates of OAT1 and OAT3.
In vitro, dolutegravir did not inhibit (IC 50 greater than 50 microM) the following: cytochrome P450 (CYP)1A2, CYP2A6, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, CYP3A, uridine diphosphate (UDP)-glucuronosyl transferase 1A1 (UGT1A1), UGT2B7, P-glycoprotein (P-gp), breast cancer resistance protein (BCRP), bile salt export pump (BSEP), organic anion transporter polypeptide (OATP)1B1, OATP1B3, OCT1, multidrug resistance protein (MRP)2, or MRP4. In vitro, dolutegravir did not induce CYP1A2, CYP2B6, or CYP3A4. Based on these data and the results of drug interaction trials, dolutegravir is not expected to affect the pharmacokinetics of drugs that are substrates of these enzymes or transporters.
7.2 Effect of Other Agents on the Pharmacokinetics of Dolutegravir
Dolutegravir is metabolized by UGT1A1 with some contribution from CYP3A. Dolutegravir is also a substrate of UGT1A3, UGT1A9, BCRP, and P-gp in vitro. Drugs that induce those enzymes and transporters may decrease dolutegravir plasma concentration and reduce the therapeutic effect of dolutegravir.
Coadministration of dolutegravir and other drugs that inhibit these enzymes may increase dolutegravir plasma concentration.
Etravirine significantly reduced plasma concentrations of dolutegravir, but the effect of etravirine was mitigated by coadministration of lopinavir/ritonavir or darunavir/ritonavir, and is expected to be mitigated by atazanavir/ritonavir ( Table 6) [see Drug Interactions ( 7.3), Clinical Pharmacology ( 12.3)] .
In vitro, dolutegravir was not a substrate of OATP1B1 or OATP1B3.
7.3 Established and Other Potentially Significant Drug Interactions
Table 6 provides clinical recommendations as a result of drug interactions with TIVICAY. These recommendations are based on either drug interaction trials or predicted interactions due to the expected magnitude of interaction and potential for serious adverse events or loss of efficacy. [See Dosage and Administration ( 2), Clinical Pharmacology ( 12.3).]
|
Concomitant Drug Class:
|
Effect on Concentration of Dolutegravir and/or Concomitant Drug |
Clinical Comment |
|
HIV-1 Antiviral Agents |
||
|
Non-nucleoside reverse transcriptase inhibitor: Etravirine a |
↓Dolutegravir |
Use of TIVICAY with etravirine without coadministration of atazanavir/ritonavir, darunavir/ritonavir, or lopinavir/ritonavir is not recommended. |
|
Non-nucleoside reverse transcriptase inhibitor: Efavirenz a |
↓Dolutegravir |
Adjust dose of TIVICAY to 50 mg twice daily for treatment-naïve and treatment-experienced, INSTI-naïve adult patients. In pediatric patients, increase the weight-based dose to twice daily ( Table 2). Use alternative combinations that do not include metabolic inducers where possible for INSTI-experienced patients with certain INSTI-associated resistance substitutions or clinically suspected INSTI resistance. b |
|
Non-nucleoside reverse transcriptase inhibitor: Nevirapine |
↓Dolutegravir |
Avoid coadministration with nevirapine because there are insufficient data to make dosing recommendations. |
|
Protease inhibitor: Fosamprenavir/ritonavir a Tipranavir/ritonavir a |
↓Dolutegravir |
Adjust dose of TIVICAY to 50 mg twice daily for treatment-naïve and treatment-experienced, INSTI-naïve adult patients. In pediatric patients, increase the weight-based dose to twice daily ( Table 2). Use alternative combinations that do not include metabolic inducers where possible for INSTI-experienced patients with certain INSTI-associated resistance substitutions or clinically suspected INSTI resistance. b |
|
Other Agents |
||
|
Dofetilide |
↑Dofetilide |
Coadministration is contraindicated with TIVICAY [see Contraindications ( 4)] . |
|
Carbamazepine a |
↓Dolutegravir |
Adjust dose of TIVICAY to 50 mg twice daily in treatment-naïve or treatment-experienced, INSTI-naïve adult patients. In pediatric patients, increase the weight-based dose to twice daily ( Table 2). Use alternative treatment that does not include carbamazepine where possible for INSTI-experienced patients with certain INSTI-associated resistance substitutions or clinically suspected INSTI resistance. b |
|
Oxcarbazepine
|
↓Dolutegravir |
Avoid coadministration with TIVICAY because there are insufficient data to make dosing recommendations. |
|
Medications containing polyvalent cations
Cation-containing antacids
a or laxatives
|
↓Dolutegravir |
Administer TIVICAY 2 hours before or 6 hours after taking medications containing polyvalent cations. |
|
Oral calcium or iron supplements, including multivitamins containing calcium or irona |
↓Dolutegravir |
Administer TIVICAY 2 hours before or 6 hours after taking supplements containing calcium or iron. Alternatively, TIVICAY and supplements containing calcium or iron can be taken together with food. |
|
Metformin |
↑Metformin |
With concomitant use, limit the total daily dose of metformin to 1,000 mg either when starting metformin or TIVICAY. When stopping TIVICAY, the metformin dose may require an adjustment. Monitoring of blood glucose when initiating concomitant use and after withdrawal of TIVICAY is recommended. |
|
Rifampin a |
↓Dolutegravir |
Adjust dose of TIVICAY to 50 mg twice daily for treatment-naïve and treatment-experienced, INSTI-naïve adult patients. In pediatric patients, increase the weight-based dose to twice daily ( Table 2). Use alternatives to rifampin where possible for INSTI-experienced patients with certain INSTI-associated resistance substitutions or clinically suspected INSTI resistance. b |
a See Clinical Pharmacology ( 12.3) Table 9 or Table 10 for magnitude of interaction.
b The lower dolutegravir exposures observed in INSTI-experienced patients (with certain INSTI-associated resistance substitutions or clinically suspected INSTI resistance [see Microbiology ( 12.4)] ) upon coadministration with certain inducers may result in loss of therapeutic effect and development of resistance to TIVICAY or other coadministered antiretroviral agents.
7.4 Drugs without Clinically Significant Interactions with Dolutegravir
Based on drug interaction trial results, the following drugs can be coadministered with dolutegravir without a dose adjustment: atazanavir/ritonavir, darunavir/ritonavir, daclatasvir, elbasvir/grazoprevir, methadone, midazolam, omeprazole, oral contraceptives containing norgestimate and ethinyl estradiol, prednisone, rifabutin, rilpivirine, sofosbuvir/velpatasvir, and tenofovir [see Clinical Pharmacology ( 12.3)] .
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Exposure Registry
There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to TIVICAY during pregnancy. Healthcare providers are encouraged to register patients by calling the Antiretroviral Pregnancy Registry (APR) at 1-800-258-4263.
Risk Summary
There are insufficient human data on the use of TIVICAY during pregnancy to inform a drug-associated risk of birth defects and miscarriage. Given the limited number of pregnancies exposed to dolutegravir-based regimens reported to the APR, no definitive conclusions can be drawn on the safety of TIVICAY in pregnancy, and continued monitoring is ongoing through the APR. The background rate for major birth defects in a U.S. reference population of the Metropolitan Atlanta Congenital Defects Program (MACDP) is 2.7%. The rate of miscarriage is not reported in the APR. The estimated background rate of miscarriage in clinically recognized pregnancies in the U.S. general population is 15% to 20%. The background risk for major birth defects and miscarriage for the indicated population is unknown. The APR uses the MACDP as the U.S. reference population for birth defects in the general population. The MACDP evaluates women and infants from a limited geographic area and does not include outcomes for births that occurred at less than 20 weeks’ gestation.
In animal reproduction studies, no evidence of adverse developmental outcomes was observed with dolutegravir (see Data). During organogenesis in the rat and rabbit, systemic exposures (AUC) to dolutegravir were less than (rabbits) and approximately 27 times (rats) the exposure in humans at the maximum recommended human dose (MRHD). In the rat pre/post-natal developmental study, maternal systemic exposure (AUC) to dolutegravir was approximately 27 times the exposure in humans at the MRHD.
Data
Animal Data: Dolutegravir was administered orally at up to 1,000 mg per kg daily to pregnant rats and rabbits on gestation Days 6 to 17 and 6 to 18, respectively, and also to rats on gestation day 6 to lactation/post-partum Day 20. No adverse effects on embryo-fetal (rats and rabbits) or pre/post-natal (rats) development were observed at up to the highest dose tested. During organogenesis systemic exposures (AUC) to dolutegravir in rabbits were less than the exposure in humans at the MRHD and in rats were approximately 27 times the exposure in humans at the MRHD. In the rat pre/post-natal development study, decreased body weight of the developing offspring was observed during lactation at a maternally toxic dose (approximately 27 times human exposure at the MRHD).
8.2 Lactation
Risk Summary
The Centers for Disease Control and Prevention recommend that HIV‑1‑infected mothers in the United States not breastfeed their infants to avoid risking postnatal transmission of HIV-1 infection. It is not known whether TIVICAY is present in human breast milk, affects human milk production, or has effects on the breastfed infant. When administered to lactating rats, dolutegravir was present in milk (see Data).
Because of the potential for (1) HIV‑1 transmission (in HIV-negative infants), and (2) developing viral resistance (in HIV-positive infants), instruct mothers not to breastfeed if they are receiving TIVICAY.
Data
Animal Data: Dolutegravir was the primary drug-related component excreted into the milk of lactating rats following a single oral dose of 50 mg per kg on lactation Day 10, with milk concentrations of up to approximately 1.3 times that of maternal plasma concentrations observed 8 hours post-dose.
8.4 Pediatric Use
The safety, virologic, and immunologic responses in subjects who received TIVICAY were evaluated in 46 treatment-experienced, INSTI-naïve, HIV‑1–infected subjects aged 6 to less than 18 years in an open-label, multicenter, dose-finding clinical trial, IMPAACT P1093 [see Clinical Pharmacology ( 12.3), Clinical Studies ( 14.2)] . Frequency, type, and severity of adverse reactions among the 46 pediatric subjects were comparable to those observed in adults [see Adverse Reactions ( 6.2)] . In 17 subjects weighing at least 30 kg, pharmacokinetic parameters of dolutegravir were comparable to adults receiving 50 mg once daily [see Clinical Pharmacology ( 12.3)].
Safety and efficacy of TIVICAY have not been established in pediatric patients weighing less than 30 kg or in any pediatric patients who are INSTI-experienced.
8.5 Geriatric Use
Clinical trials of TIVICAY did not include sufficient numbers of subjects aged 65 and older to determine whether they respond differently from younger subjects. In general, caution should be exercised in the administration of TIVICAY in elderly patients reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy [see Clinical Pharmacology ( 12.3)] .
8.6 Hepatic Impairment
No clinically important pharmacokinetic differences between subjects with moderate hepatic impairment and matching healthy subjects were observed. No dosage adjustment is necessary for patients with mild to moderate hepatic impairment (Child-Pugh Score A or B). The effect of severe hepatic impairment (Child-Pugh Score C) on the pharmacokinetics of dolutegravir has not been studied. Therefore, TIVICAY is not recommended for use in patients with severe hepatic impairment [see Clinical Pharmacology ( 12.3)] .
8.7 Renal Impairment
Dolutegravir plasma concentrations were decreased in subjects with severe renal impairment compared with those in matched healthy controls. However, no dosage adjustment is necessary for treatment-naïve or treatment-experienced and INSTI-naïve patients with mild, moderate, or severe renal impairment or for INSTI-experienced patients (with certain INSTI-associated resistance substitutions or clinically suspected INSTI resistance) with mild or moderate renal impairment. Caution is warranted for INSTI-experienced patients (with certain INSTI-associated resistance substitutions or clinically suspected INSTI resistance [see Microbiology ( 12.4)] ) with severe renal impairment, as the decrease in dolutegravir concentrations may result in loss of therapeutic effect and development of resistance to TIVICAY or other coadministered antiretroviral agents [see Clinical Pharmacology ( 12.3)] . Dolutegravir has not been studied in patients on dialysis.
10 OVERDOSAGE
There is no known specific treatment for overdose with TIVICAY. If overdose occurs, the patient should be monitored and standard supportive treatment applied as required. As dolutegravir is highly bound to plasma proteins, it is unlikely that it will be significantly removed by dialysis.
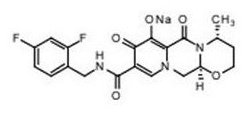
11 DESCRIPTION
TIVICAY contains dolutegravir, as dolutegravir sodium, an HIV INSTI. The chemical name of dolutegravir sodium is sodium (4 R,12a S)-9-{[(2,4-difluorophenyl)methyl]carbamoyl}-4-methyl-6,8-dioxo-3,4,6,8,12,12a-hexahydro-2 H-pyrido[1',2':4,5]pyrazino[2,1- b][1,3]oxazin-7-olate. The empirical formula is C 20H 18F 2N 3NaO 5 and the molecular weight is 441.36 g per mol. It has the following structural formula:
Dolutegravir sodium is a white to light yellow powder and is slightly soluble in water.
Each film-coated tablet of TIVICAY for oral administration contains 10.5, 26.3, or 52.6 mg of dolutegravir sodium, which is equivalent to 10, 25, or 50 mg dolutegravir free acid, respectively, and the following inactive ingredients: D-mannitol, microcrystalline cellulose, povidone K29/32, sodium starch glycolate, and sodium stearyl fumarate. The tablet film‑coating contains the inactive ingredients iron oxide yellow (25-mg and 50-mg tablets only), macrogol/PEG, polyvinyl alcohol-part hydrolyzed, talc, and titanium dioxide.
12 CLINICAL PHARMACOLOGY
12.2 Pharmacodynamics
Effects on Electrocardiogram
In a randomized, placebo-controlled, cross-over trial, 42 healthy subjects received single-dose oral administrations of placebo, dolutegravir 250-mg suspension (exposures approximately 3–fold of the 50-mg once-daily dose at steady state), and moxifloxacin 400 mg (active control) in random sequence. After baseline and placebo adjustment, the maximum mean QTc change based on Fridericia correction method (QTcF) for dolutegravir was 2.4 msec (1-sided 95% upper CI: 4.9 msec). TIVICAY did not prolong the QTc interval over 24 hours` post-dose.
Effects on Renal Function
The effect of dolutegravir on renal function was evaluated in an open-label, randomized, 3‑arm, parallel, placebo-controlled trial in healthy subjects (n = 37) who received dolutegravir 50 mg once daily (n = 12), dolutegravir 50 mg twice daily (n = 13), or placebo once daily (n = 12) for 14 days. A decrease in creatinine clearance, as determined by 24-hour urine collection, was observed with both doses of dolutegravir after 14 days of treatment in subjects who received 50 mg once daily (9% decrease) and 50 mg twice daily (13% decrease). Neither dose of dolutegravir had a significant effect on the actual glomerular filtration rate (determined by the clearance of probe drug, iohexol) or effective renal plasma flow (determined by the clearance of probe drug, para-amino hippurate) compared with the placebo.
12.3 Pharmacokinetics
The pharmacokinetic properties of dolutegravir have been evaluated in healthy adult subjects and HIV‑1–infected adult subjects. Exposure to dolutegravir was generally similar between healthy subjects and HIV‑1–infected subjects. The non-linear exposure of dolutegravir following 50 mg twice daily compared with 50 mg once daily in HIV‑1–infected subjects ( Table 7) was attributed to the use of metabolic inducers in the background antiretroviral regimens of subjects receiving dolutegravir 50 mg twice daily in clinical trials.
|
Parameter |
50 mg Once Daily Geometric Mean a (%CV) |
50 mg Twice Daily Geometric Mean b (%CV) |
|
AUC (0-24) (mcg.h/mL) |
53.6 (27) |
75.1 (35) |
|
C max (mcg/mL) |
3.67 (20) |
4.15 (29) |
|
C min (mcg/mL) |
1.11 (46) |
2.12 (47) |
a Based on population pharmacokinetic analyses using data from SPRING-1 and SPRING-2.
b Based on population pharmacokinetic analyses using data from VIKING (ING112961) and VIKING-3.
Absorption
Following oral administration of dolutegravir, peak plasma concentrations were observed 2 to 3 hours postdose. With once-daily dosing, pharmacokinetic steady state is achieved within approximately 5 days with average accumulation ratios for AUC, C max, and C 24 h ranging from 1.2 to 1.5.
Dolutegravir plasma concentrations increased in a less than dose-proportional manner above 50 mg. Dolutegravir is a P‑gp substrate in vitro. The absolute bioavailability of dolutegravir has not been established.
Effect of Food: TIVICAY may be taken with or without food. Food increased the extent of absorption and slowed the rate of absorption of dolutegravir. Low-, moderate-, and high-fat meals increased dolutegravir AUC (0-∞) by 33%, 41%, and 66%; increased C max by 46%, 52%, and 67%; and prolonged T max to 3, 4, and 5 hours from 2 hours under fasted conditions, respectively.
Distribution
Dolutegravir is highly bound (greater than or equal to 98.9%) to human plasma proteins based on in vivo data and binding is independent of plasma concentration of dolutegravir. The apparent volume of distribution (Vd/F) following 50-mg once-daily administration is estimated at 17.4 L based on a population pharmacokinetic analysis.
Cerebrospinal Fluid (CSF): In 12 treatment-naïve subjects on dolutegravir 50 mg daily plus abacavir/lamivudine, the median dolutegravir concentration in CSF was 13.2 ng per mL (range: 3.74 ng per mL to 18.3 ng per mL) 2 to 6 hours postdose after 16 weeks of treatment. The clinical relevance of this finding has not been established.
Elimination
Dolutegravir has a terminal half-life of approximately 14 hours and an apparent clearance (CL/F) of 1.0 L per hour based on population pharmacokinetic analyses.
Metabolism: Dolutegravir is primarily metabolized via UGT1A1 with some contribution from CYP3A.
Polymorphisms in Drug‑Metabolizing Enzymes: In a meta-analysis of healthy subject trials, subjects with UGT1A1 (n = 7) genotypes conferring poor dolutegravir metabolism had a 32% lower clearance of dolutegravir and 46% higher AUC compared with subjects with genotypes associated with normal metabolism via UGT1A1 (n = 41).
Excretion: After a single oral dose of [ 14C] dolutegravir, 53% of the total oral dose was excreted unchanged in feces. Thirty-one percent of the total oral dose was excreted in urine, represented by an ether glucuronide of dolutegravir (18.9% of total dose), a metabolite formed by oxidation at the benzylic carbon (3.0% of total dose), and its hydrolytic N-dealkylation product (3.6% of total dose). Renal elimination of unchanged drug was low (less than 1% of the dose).
Specific Populations
Pediatric Patients: The pharmacokinetics of dolutegravir in HIV‑1–infected children (n = 17) weighing at least 30 kg (dosed by weight bands, receiving either 35 mg or 50 mg) were similar to those observed in HIV‑1–infected adults who received dolutegravir 50 mg once daily ( Table 8) [see Clinical Studies ( 14.2)] .
|
Weight (n) |
Dose of TIVICAY |
Dolutegravir Pharmacokinetic Parameter Estimates Geometric Mean (%CV) |
||
|
C max (mcg/mL) |
AUC (0-24) (mcg.h/mL) |
C 24 (mcg/mL) |
||
|
≥40 kg (n = 14) |
50 mg once daily |
3.89 (43) |
50.1 (53) |
0.99 (66) |
|
≥30 to <40 kg
|
35 mg once daily |
4.40 (54) |
64.6 (64) |
1.33 (93) |
Geriatric Patients: Population pharmacokinetic analysis indicated age had no clinically relevant effect on the pharmacokinetics of dolutegravir.
Patients with Hepatic Impairment: In a trial comparing 8 subjects with moderate hepatic impairment (Child-Pugh Score B) with 8 matched healthy controls, exposure of dolutegravir from a single 50-mg dose was similar between the 2 groups. The effect of severe hepatic impairment (Child-Pugh Score C) on the pharmacokinetics of dolutegravir has not been studied.
Patients with Renal Impairment: In a trial comparing 8 subjects with severe renal impairment (CrCl less than 30 mL per min) with 8 matched healthy controls, AUC, C max, and C 24 of dolutegravir were lower by 40%, 23%, and 43%, respectively, compared with those in matched healthy subjects. Population pharmacokinetic analysis using data from SAILING and VIKING-3 trials indicated that mild and moderate renal impairment had no clinically relevant effect on the exposure of dolutegravir. Dolutegravir has not been studied in patients requiring dialysis.
HBV or HCV Co-infected Patients: Population analyses using pooled pharmacokinetic data from adult trials indicated no clinically relevant effect of HCV co-infection on the pharmacokinetics of dolutegravir. There were limited data on HBV co-infection.
Gender and Race: Population analyses using pooled pharmacokinetic data from adult trials indicated gender and race had no clinically relevant effect on the exposure of dolutegravir.
Drug Interaction Studies
Drug interaction trials were performed with TIVICAY and other drugs likely to be coadministered or commonly used as probes for pharmacokinetic interactions. The effects of dolutegravir on the exposure of coadministered drugs are summarized in Table 9 and the effects of coadministered drugs on the exposure of dolutegravir are summarized in Table 10.
Dosing or regimen recommendations as a result of established and other potentially significant drug-drug interactions with TIVICAY are provided in Table 6 [see Dosage and Administration ( 2.1), Drug Interactions ( 7.3)] .
|
Coadministered Drug(s) and Dose(s) |
Dose of TIVICAY |
n |
Geometric Mean Ratio (90% CI) of Pharmacokinetic Parameters of Coadministered Drug with/without Dolutegravir
|
||
|
C max |
AUC |
C τ or C 24 |
|||
|
Daclatasvir
|
50 mg
|
12 |
1.03
|
0.98
|
1.06
|
|
Elbasvir
|
50 mg single dose |
12 |
0.97 (0.89, 1.05) |
0.98 (0.93, 1.04) |
0.98 (0.93, 1.03) |
|
Ethinyl estradiol
|
50 mg
|
15 |
0.99
|
1.03
|
1.02
|
|
Grazoprevir 200 mg once daily |
50 mg single dose |
12 |
0.64 (0.44, 0.93) |
0.81 (0.67, 0.97) |
0.86 (0.79, 0.93) |
|
Metformin
|
50 mg
|
15 a |
1.66
|
1.79
|
_ |
|
Metformin
|
50 mg
|
15 a |
2.11
|
2.45
|
_ |
|
Methadone
|
50 mg
|
11 |
1.00
|
0.98
|
0.99
|
|
Midazolam
|
25 mg
|
10 |
_ |
0.95
|
_ |
|
Norelgestromin
|
50 mg
|
15 |
0.89
|
0.98
|
0.93
|
|
Rilpivirine
|
50 mg
|
16 |
1.10
|
1.06
|
1.21
|
|
Sofosbuvir 400 mg once daily Metabolite (GS-331007) |
50 mg once daily |
24 |
0.88 (0.80, 0.98) |
0.92 (0.85, 0.99) |
NA |
|
1.01 (0.93, 1.10) |
0.99 (0.97, 1.01) |
0.99 (0.97, 1.01) |
|||
|
Tenofovir disoproxil fumarate
|
50 mg
|
15 |
1.09
|
1.12
|
1.19
|
|
Velpatasvir 100 mg once daily |
50 mg once daily |
24 |
0.94 (0.86, 1.02) |
0.91 (0.84, 0.98) |
0.88 (0.82, 0.94) |
a The number of subjects represents the maximum number of subjects that were evaluated.
|
Coadministered Drug(s) and Dose(s) |
Dose of TIVICAY |
n |
Geometric Mean Ratio (90% CI) of Dolutegravir Pharmacokinetic Parameters with/without Coadministered Drugs
|
||
|
C max |
AUC |
C τ or C 24 |
|||
|
Atazanavir
|
30 mg
|
12 |
1.50
|
1.91
|
2.80
|
|
Atazanavir/ritonavir
|
30 mg
|
12 |
1.34
|
1.62
|
2.21
|
|
Darunavir/ritonavir
|
30 mg
|
15 |
0.89
|
0.78
|
0.62
|
|
Efavirenz
|
50 mg
|
12 |
0.61
|
0.43
|
0.25
|
|
Elbasvir/grazoprevir 50/200 mg once daily |
50 mg single dose |
12 |
1.22 (1.05, 1.40) |
1.16 (1.00, 1.34) |
1.14 (0.95, 1.36) |
|
Etravirine
|
50 mg
|
16 |
0.48
|
0.29
|
0.12
|
|
Etravirine + darunavir/ritonavir
|
50 mg
|
9 |
0.88
|
0.75
|
0.63
|
|
Etravirine + lopinavir/ritonavir
|
50 mg
|
8 |
1.07
|
1.11
|
1.28
|
|
Fosamprenavir/ritonavir
|
50 mg
|
12 |
0.76
|
0.65
|
0.51
|
|
Lopinavir/ritonavir
|
30 mg
|
15 |
1.00
|
0.97
|
0.94
|
|
Rilpivirine
|
50 mg
|
16 |
1.13
|
1.12
|
1.22
|
|
Tenofovir
|
50 mg
|
15 |
0.97
|
1.01
|
0.92 (0.82 to 1.04) |
|
Tipranavir/ritonavir
|
50 mg
|
14 |
0.54
|
0.41
|
0.24
|
|
Antacid (MAALOX)
|
50 mg
|
16 |
0.28
|
0.26
|
0.26
|
|
Antacid (MAALOX)
|
50 mg
|
16 |
0.82
|
0.74
|
0.70
|
|
Calcium carbonate 1,200 mg
|
50 mg
|
12 |
0.63
|
0.61
|
0.61
|
|
Calcium carbonate 1,200 mg
|
50 mg
|
11 |
1.07
|
1.09
|
1.08
|
|
Calcium carbonate 1,200 mg
|
50 mg
|
11 |
1.00
|
0.94
|
0.90
|
|
Carbamazepine
|
50 mg
|
16 c |
0.67
|
0.51
|
0.27
|
|
Daclatasvir
|
50 mg
|
12 |
1.29
|
1.33
|
1.45
|
|
Ferrous fumarate 324 mg
|
50 mg
|
11 |
0.43
|
0.46
|
0.44
|
|
Ferrous fumarate 324 mg
|
50 mg
|
11 |
1.03
|
0.98
|
1.00
|
|
Ferrous fumarate 324 mg
|
50 mg
|
10 |
0.99
|
0.95
|
0.92
|
|
Multivitamin (One-A-Day)
|
50 mg
|
16 |
0.65
|
0.67
|
0.68
|
|
Omeprazole
|
50 mg
|
12 |
0.92
|
0.97
|
0.95
|
|
Prednisone
|
50 mg
|
12 |
1.06
|
1.11
|
1.17
|
|
Rifampin
a
|
50 mg
|
11 |
0.57
|
0.46
|
0.28
|
|
Rifampin
b
|
50 mg
|
11 |
1.18
|
1.33
|
1.22
|
|
Rifabutin
|
50 mg
|
9 |
1.16
|
0.95
|
0.70
|
a Comparison is rifampin taken with dolutegravir 50 mg twice daily compared with dolutegravir 50 mg twice daily.
b Comparison is rifampin taken with dolutegravir 50 mg twice daily compared with dolutegravir 50 mg once daily.
c The number of subjects represents the maximum number of subjects that were evaluated.
12.4 Microbiology
Mechanism of Action
Dolutegravir inhibits HIV integrase by binding to the integrase active site and blocking the strand transfer step of retroviral deoxyribonucleic acid (DNA) integration which is essential for the HIV replication cycle. Strand transfer biochemical assays using purified HIV-1 integrase and pre-processed substrate DNA resulted in IC 50 values of 2.7 nM and 12.6 nM.
Antiviral Activity in Cell Culture
Dolutegravir exhibited antiviral activity against laboratory strains of wild-type HIV-1 with mean EC 50 values of 0.5 nM (0.21 ng per mL) to 2.1 nM (0.85 ng per mL) in peripheral blood mononuclear cells (PBMCs) and MT-4 cells. Dolutegravir exhibited antiviral activity against 13 clinically diverse clade B isolates with a mean EC 50 value of 0.52 nM in a viral integrase susceptibility assay using the integrase coding region from clinical isolates. Dolutegravir demonstrated antiviral activity in cell culture against a panel of HIV-1 clinical isolates (3 in each group of M clades A, B, C, D, E, F, and G, and 3 in group O) with EC 50 values ranging from 0.02 nM to 2.14 nM for HIV-1. Dolutegravir EC 50 values against 3 HIV-2 clinical isolates in PBMC assays ranged from 0.09 nM to 0.61 nM.
Antiviral Activity in Combination with Other Antiviral Agents
The antiviral activity of dolutegravir was not antagonistic when combined with the INSTI, raltegravir; non-nucleoside reverse transcriptase inhibitors (NNRTIs), efavirenz or nevirapine; the nucleoside reverse transcriptase inhibitors (NRTIs), abacavir or stavudine; the protease inhibitors (PIs), amprenavir or lopinavir; the CCR5 co-receptor antagonist, maraviroc; or the fusion inhibitor, enfuvirtide. Dolutegravir antiviral activity was not antagonistic when combined with the HBV reverse transcriptase inhibitor, adefovir, or inhibited by the antiviral, ribavirin.
Resistance
Cell Culture: Dolutegravir-resistant viruses were selected in cell culture starting from different wild-type HIV-1 strains and clades. Amino acid substitutions E92Q, G118R, S153F or Y, G193E or R263K emerged in different passages and conferred decreased susceptibility to dolutegravir of up to 4-fold. Passage of mutant viruses containing the Q148R or Q148H substitutions selected for additional substitutions in integrase that conferred decreased susceptibility to dolutegravir (fold-change increase of 13 to 46). The additional integrase substitutions included T97A, E138K, G140S, and M154I. Passage of mutant viruses containing both G140S and Q148H selected for L74M, E92Q, and N155H.
Treatment-Naïve Subjects: No subjects in the dolutegravir 50-mg once-daily treatment arms of treatment-naïve trials SPRING-2 (96 weeks) and SINGLE (144 weeks) had a detectable decrease in susceptibility to dolutegravir or background NRTIs in the resistance analysis subset (n = 12 with HIV-1 RNA greater than 400 copies per mL at failure or last visit and having resistance data). Two virologic failure subjects in SINGLE had treatment-emergent G/D/E193D and G193G/E integrase substitutions at Week 84 and Week 108, respectively, and 1 subject with 275 copies per mL HIV-1 RNA had a treatment-emergent Q157Q/P integrase substitution detected at Week 24. None of these subjects had a corresponding decrease in dolutegravir susceptibility. No treatment-emergent genotypic resistance to the background regimen was observed in the dolutegravir arm in either the SPRING-2 or SINGLE trials. No treatment-emergent primary resistance substitutions were observed in either treatment group in the FLAMINGO trial through Week 96.
Treatment-Experienced, Integrase Strand Transfer Inhibitor-Naïve Subjects: In the dolutegravir arm of the SAILING trial for treatment-experienced and INSTI-naïve subjects (n = 354), treatment-emergent integrase substitutions were observed in 6 of 28 (21%) subjects who had virologic failure and resistance data. In 5 of the 6 subjects’ isolates emergent INSTI substitutions included L74L/M/I, Q95Q/L, V151V/I (n = 1 each), and R263K (n = 2). The change in dolutegravir phenotypic susceptibility for these 5 subject isolates was less than 2-fold. One subject isolate had pre-existing raltegravir resistance substitutions E138A, G140S, and Q148H at baseline and had additional emergent INSTI-resistance substitutions T97A and E138A/T with a corresponding 148-fold reduction in dolutegravir susceptibility at failure. In the comparator raltegravir arm, 21 of 49 (43%) subjects with post-baseline resistance data had evidence of emergent INSTI-resistance substitutions (L74M, E92Q, T97A, E138Q, G140S/A, Y143R/C, Q148H/R, V151I, N155H, E157Q, and G163K/R) and raltegravir phenotypic resistance.
Virologically Suppressed Subjects: SWORD-1 and SWORD-2 are identical trials in virologically suppressed subjects receiving 2 NRTIs plus either an INSTI, an NNRTI, or a PI, that switched to dolutegravir plus rilpivirine (n = 513) or remained on their current antiviral regimen (n = 511). Two subjects in each treatment arm had confirmed virologic failure at any time through Week 48. The 2 subjects in the dolutegravir/rilpivirine arm had detectable resistance substitutions at rebound. One subject had the NNRTI-resistance-associated substitution K101K/E with no decreased susceptibility to rilpivirine (fold-change = 1.2) at Week 36, had no INSTI resistance-associated substitutions or decreased susceptibility to dolutegravir (fold-change less than 2), and had HIV-1 RNA less than 50 copies per mL at the withdrawal visit. The other subject had the dolutegravir resistance-associated substitution G193E at baseline (by exploratory HIV proviral DNA archive sequencing) and at Week 24 (by conventional sequencing) without decreased susceptibility to dolutegravir (fold-change = 1.02) at Week 24. No resistance-associated substitutions were observed for the other 2 subjects in the comparative current antiretroviral regimen arm.
Treatment-Experienced, Integrase Strand Transfer Inhibitor-Experienced Subjects: VIKING-3 examined the efficacy of dolutegravir 50 mg twice daily plus optimized background therapy in subjects with prior or current virologic failure on an INSTI- (elvitegravir or raltegravir) containing regimen. Use of TIVICAY in INSTI-experienced patients should be guided by the number and type of baseline INSTI substitutions. The efficacy of TIVICAY 50 mg twice daily is reduced in patients with an INSTI-resistance Q148 substitution plus 2 or more additional INSTI-resistance substitutions, including T66A, L74I/M, E138A/K/T, G140S/A/C, Y143R/C/H, E157Q, G163S/E/K/Q, or G193E/R.
Response by Baseline Genotype
Of the 183 subjects with baseline data, 30% harbored virus with a substitution at Q148, and 33% had no primary INSTI-resistance substitutions (T66A/I/K, E92Q/V, Y143R/C/H, Q148H/R/K, and N155H) at baseline, but had historical genotypic evidence of INSTI-resistance substitutions, phenotypic evidence of elvitegravir or raltegravir resistance, or genotypic evidence of INSTI-resistance substitutions at screening.
Response rates by baseline genotype were analyzed in an “as-treated” analysis at Week 48 (n = 175) ( Table 11). The response rate at Week 48 to dolutegravir-containing regimens was 47% (24 of 51) when Q148 substitutions were present at baseline; Q148 was always present with additional INSTI-resistance substitutions (see Table 11). In addition, a diminished virologic response of 40% (6 of 15) was observed when the substitution E157Q or K was present at baseline with other INSTI-resistance substitutions but without a Q148H or R substitution.
|
Baseline Genotype |
Week 48
|
|
Overall Response |
66% (116/175) |
|
No Q148 substitution a |
74% (92/124) |
|
Q148H/R + G140S/A/C without additional INSTI-resistance substitution b |
61% (17/28) |
|
Q148H/R + ≥2 INSTI-resistance substitutions b,c |
29% (6/21) |
a Includes INSTI-resistance substitutions Y143R/C/H and N155H.
b INSTI-resistance substitutions included T66A, L74I/M, E138A/K/T, G140S/A/C, Y143R/C/H, E157Q, G163S/E/K/Q, or G193E/R. Two additional subjects had baseline genotypes of Q148Q/R plus L74L/I/M (virologic failure) and Q148R plus E138K (responder).
c The most common pathway with Q148H/R + greater than or equal to 2 INSTI-resistance substitutions had Q148+G140+E138 substitutions (n = 16).
Response by Baseline Phenotype
Response rates by baseline phenotype were analyzed in an as-treated analysis using all subjects with available baseline phenotypes through Week 48 (n = 163) (see Table 12). These baseline phenotypic groups are based on subjects enrolled in VIKING-3 and are not meant to represent definitive clinical susceptibility cut points for dolutegravir. The data are provided to guide clinicians on the likelihood of virologic success based on pretreatment susceptibility to dolutegravir in INSTI-resistant patients.
|
Baseline Dolutegravir Phenotype
|
Response at Week 48
|
|
Overall Response |
64% (104/163) |
|
<3-fold change |
72% (83/116) |
|
3- <10-fold change |
53% (18/34) |
|
≥10-fold change |
23% (3/13) |
Integrase Strand Transfer Inhibitor Treatment-Emergent Resistance
There were 50 subjects with virologic failure on the dolutegravir twice-daily regimen in VIKING-3 with HIV-1 RNA greater than 400 copies per mL at the failure timepoint, Week 48 or beyond, or the last timepoint on trial. Thirty-nine subjects with virologic failure had resistance data that were used in the Week 48 analysis. In the Week 48 resistance analysis 85% (33 of 39) of the subjects with virologic failure had treatment-emergent INSTI-resistance substitutions in their isolates. The most common treatment-emergent INSTI-resistance substitution was T97A. Other frequently emergent INSTI-resistance substitutions included L74M, I or V, E138K or A, G140S, Q148H, R or K, M154I, or N155H. Substitutions E92Q, Y143R or C/H, S147G, V151A, and E157E/Q each emerged in 1 to 3 subjects’ isolates. At failure, the median dolutegravir fold-change from reference was 61-fold (range: 0.75 to 209) for isolates with emergent INSTI-resistance substitutions (n = 33).
Resistance to one or more background drugs in the dolutegravir twice-daily regimen also emerged in 49% (19 of 39) subjects in the Week 48 resistance analysis .
In VIKING-4 (ING116529), 30 subjects with current virological failure on an INSTI-containing regimen and genotypic evidence of INSTI-resistance substitutions at screening were randomized to receive either dolutegravir 50 mg twice daily or placebo with the current failing regimen for 7 days and then all subjects received open-label dolutegravir plus optimized background regimen from Day 8. Virologic responses at Week 48 by baseline genotypic and phenotypic INSTI-resistance categories and the INSTI resistance-associated substitutions that emerged on dolutegravir treatment in VIKING-4 were consistent with those seen in VIKING-3.
Cross-Resistance
Site-Directed Integrase Strand Transfer Inhibitor-Resistant Mutant HIV-1 and HIV-2 Strains: The susceptibility of dolutegravir was tested against 60 INSTI-resistant site-directed mutant HIV-1 viruses (28 with single substitutions and 32 with 2 or more substitutions) and 6 INSTI-resistant site-directed mutant HIV-2 viruses. The single INSTI-resistance substitutions T66K, I151L, and S153Y conferred a greater than 2-fold decrease in dolutegravir susceptibility (range: 2.3-fold to 3.6-fold from reference). Combinations of multiple substitutions T66K/L74M, E92Q/N155H, G140C/Q148R, G140S/Q148H, R or K, Q148R/N155H, T97A/G140S/Q148, and substitutions at E138/G140/Q148 showed a greater than 2-fold decrease in dolutegravir susceptibility (range: 2.5-fold to 21-fold from reference). In HIV-2 mutants, combinations of substitutions A153G/N155H/S163G and E92Q/T97A/N155H/S163D conferred 4-fold decreases in dolutegravir susceptibility, and E92Q/N155H and G140S/Q148R showed 8.5-fold and 17-fold decreases in dolutegravir susceptibility, respectively.
Reverse Transcriptase Inhibitor- and Protease Inhibitor-Resistant Strains: Dolutegravir demonstrated equivalent antiviral activity against 2 NNRTI-resistant, 3 NRTI-resistant, and 2 PI-resistant HIV-1 mutant clones compared with the wild-type strain.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Two-year carcinogenicity studies in mice and rats were conducted with dolutegravir. Mice were administered doses of up to 500 mg per kg, and rats were administered doses of up to 50 mg per kg. In mice, no significant increases in the incidence of drug-related neoplasms were observed at the highest doses tested, resulting in dolutegravir AUC exposures approximately 14 times higher than those in humans at the recommended dose of 50 mg twice daily. In rats, no increases in the incidence of drug-related neoplasms were observed at the highest dose tested, resulting in dolutegravir AUC exposures 10 times and 15 times higher in males and females, respectively, than those in humans at the recommended dose of 50 mg twice daily.
Mutagenesis
Dolutegravir was not genotoxic in the bacterial reverse mutation assay, mouse lymphoma assay, or in the in vivo rodent micronucleus assay.
Impairment of Fertility
In a study conducted in rats, there were no effects on mating or fertility with dolutegravir up to 1,000 mg per kg per day. This dose is associated with an exposure that is approximately 24 times higher than the exposure in humans at the recommended dose of 50 mg twice daily.
14 CLINICAL STUDIES
14.1 Description of Clinical Studies
The efficacy and safety of TIVICAY were evaluated in the studies summarized in Table 13.
|
Population |
Trial |
Trial Arms |
Timepoint (Week) |
|
Adults: | |||
|
SPRING-2 (ING113086)
|
TIVICAY + 2 NRTIs (n = 403) Raltegravir +3 NRTIs (n = 405) |
96 |
|
SINGLE (ING114467)
|
TIVICAY + EPZICOM (n = 414) ATRIPLA (n = 419) |
144 |
|
|
FLAMINGO (ING114915)
|
TIVICAY + NRTI BR (n = 243) Darunavir/ritonavir + NRTI BR (n = 242) |
96 |
|
|
SAILING (ING111762)
|
TIVICAY + BR (n = 354) Raltegravir + BR (n = 361) |
48 |
|
VIKING-3 (ING112574) (NCT01328041) |
TIVICAY + OBT (n = 183) |
48 |
|
SWORD-1 (NCT02429791) SWORD-2 (NCT02422797) |
Pooled presentation TIVICAY + Rilpivirine (n = 513) CAR (n = 511) |
48 |
|
Pediatrics:
|
IMPAACT P1093 (NCT01302847) |
TIVICAY + BR (n = 46) |
48 |
BR = Background regimen; CAR = Current antiretroviral regimen; OBT = Optimized background therapy
14.2 Adult Subjects
Treatment-Naïve Subjects
In SPRING-2, 822 subjects were randomized and received at least 1 dose of either TIVICAY 50 mg once daily or raltegravir 400 mg twice daily, both in combination with fixed-dose dual NRTI treatment (either abacavir sulfate and lamivudine [EPZICOM] or emtricitabine/tenofovir [TRUVADA]). There were 808 subjects included in the efficacy and safety analyses. At baseline, the median age of subjects was 36 years, 13% female, 15% non-white, 11% had hepatitis B and/or C virus co-infection, 2% were CDC Class C (AIDS), 28% had HIV-1 RNA greater than 100,000 copies per mL, 48% had CD4+ cell count less than 350 cells per mm 3, and 39% received EPZICOM; these characteristics were similar between treatment groups.
In SINGLE, 833 subjects were randomized and received at least 1 dose of either TIVICAY 50 mg once daily with fixed-dose abacavir sulfate and lamivudine (EPZICOM) or fixed-dose efavirenz/emtricitabine/tenofovir (ATRIPLA). At baseline, the median age of subjects was 35 years, 16% female, 32% non-white, 7% had hepatitis C co-infection (hepatitis B virus co-infection was excluded), 4% were CDC Class C (AIDS), 32% had HIV-1 RNA greater than 100,000 copies per mL, and 53% had CD4+ cell count less than 350 cells per mm 3; these characteristics were similar between treatment groups.
Outcomes for SPRING-2 (Week 96 analysis) and SINGLE (Week 144 open-label phase analysis which followed the Week 96 double-blind phase) are provided in Table 14. Side-by-side tabulation is to simplify presentation; direct comparisons across trials should not be made due to differing trial designs.
|
SPRING-2
|
SINGLE
|
|||
|
TIVICAY
|
Raltegravir
|
TIVICAY
|
ATRIPLA Once Daily
|
|
|
HIV-1 RNA <50 copies/mL |
82% |
78% |
71% |
63% |
|
4.9% (95% CI: -0.6%, 10.3%) d |
8.3% (95% CI: 2.0%, 14.6%) e |
||
|
Virologic nonresponse |
5% |
10% |
10% |
7% |
|
1% |
3% |
4% |
<1% |
|
2% |
3% |
3% |
3% |
|
<1% |
3% |
3% |
4% |
|
<1% |
<1% |
0 |
0 |
|
No virologic data |
12% |
12% |
18% |
30% |
|
Reasons | ||||
|
2% |
2% |
4% |
14% |
|
8% |
9% |
12% |
13% |
|
2% |
<1% |
2% |
3% |
|
Proportion (%) of Subjects with HIV-1 RNA <50 copies/mL by Baseline Category |
||||
|
Plasma viral load (copies/mL) | ||||
|
84% |
83% |
73% |
64% |
|
79% |
63% |
69% |
61% |
|
Gender | ||||
|
84% |
79% |
72% |
66% |
|
70% |
68% |
69% |
48% |
|
Race | ||||
|
83% |
78% |
72% |
71% |
|
77% |
75% |
71% |
47% |
a Adjusted for pre-specified stratification factors.
b Includes subjects who discontinued due to an adverse event or death at any time point if this resulted in no virologic data on treatment during the analysis window.
c Other includes reasons such as withdrew consent, loss to follow-up, moved, and protocol deviation.
d The primary endpoint was assessed at Week 48 and the virologic success rate was 88% in the group receiving TIVICAY and 86% in the raltegravir group, with a treatment difference of 2.6% and 95% CI of (-1.9%, 7.2%).
e The primary endpoint was assessed at Week 48 and the virologic success rate was 88% in the group receiving TIVICAY and 81% in the ATRIPLA group, with a treatment difference of 7.4% and 95% CI of (2.5%, 12.3%).
SPRING-2: Virologic outcomes were also comparable across baseline characteristics including CD4+ cell count, age, and use of EPZICOM or TRUVADA as NRTI background regimen. The median change in CD4+ cell counts from baseline was 276 cells per mm 3 in the group receiving TIVICAY and 264 cells per mm 3 for the raltegravir group at 96 weeks.
There was no treatment-emergent resistance to dolutegravir or to the NRTI background.
SINGLE: Treatment differences were maintained across baseline characteristics including baseline viral load, CD4+ cell count, age, gender, and race.
The adjusted mean changes in CD4+ cell counts from baseline were 378 cells per mm 3 in the group receiving TIVICAY + EPZICOM and 332 cells per mm 3 for the ATRIPLA group at 144 weeks. The adjusted difference between treatment arms and 95% CI was 46.9 cells per mm 3 (15.6 cells per mm 3, 78.2 cells per mm 3) (adjusted for pre-specified stratification factors: baseline HIV-1 RNA, and baseline CD4+ cell count).
There was no treatment-emergent resistance to dolutegravir, abacavir, or lamivudine.
FLAMINGO: In FLAMINGO, 485 subjects were randomized and received at least 1 dose of either TIVICAY 50 mg once daily (n = 243) or darunavir + ritonavir 800 mg/100 mg once daily (n = 242), both in combination with investigator-selected NRTI background regimen (either fixed-dose abacavir and lamivudine [EPZICOM] or fixed-dose emtricitabine/tenofovir disoproxil fumarate [TRUVADA]). There were 484 subjects included in the efficacy and safety analyses. At baseline, the median age of subjects was 34 years, 15% female, 28% non-white, 10% had hepatitis B and/or C virus co-infection, 3% were CDC Class C (AIDS), 25% had HIV‑1 RNA greater than 100,000 copies per mL, and 35% had CD4+ cell count less than 350 cells per mm 3; these characteristics were similar between treatment groups. Overall response rates by Snapshot algorithm through Week 96 were 80% for TIVICAY and 68% for darunavir/ritonavir. The proportion of subjects who were non-responders (HIV-1 RNA greater than or equal to 50 copies per mL) at Week 96 was 8% and 12% in the arms receiving TIVICAY and darunavir + ritonavir, respectively; no virologic data were available for 12% and 21% for subjects treated with TIVICAY and darunavir + ritonavir, respectively. The adjusted overall response rate difference in proportion and 95% CI was 12.4% (4.7%, 20.2%). No treatment-emergent primary resistance substitutions were observed in either treatment group.
Treatment-Experienced, Integrase Strand Transfer Inhibitor-Naïve Subjects
In the international, multicenter, double-blind trial (SAILING), 719 HIV‑1‑infected, antiretroviral treatment-experienced adults were randomized and received either TIVICAY 50 mg once daily or raltegravir 400 mg twice daily with investigator-selected background regimen consisting of up to 2 agents, including at least 1 fully active agent. There were 715 subjects included in the efficacy and safety analyses. At baseline, the median age was 43 years, 32% were female, 50% non-white, 16% had hepatitis B and/or C virus co-infection, 46% were CDC Class C (AIDS), 20% had HIV-1 RNA greater than 100,000 copies per mL, and 72% had CD4+ cell count less than 350 cells per mm 3; these characteristics were similar between treatment groups. All subjects had at least 2-class antiretroviral treatment resistance, and 49% of subjects had at least 3-class antiretroviral treatment resistance at baseline. Week 48 outcomes for SAILING are shown in Table 15.
|
TIVICAY 50 mg
(n = 354) |
Raltegravir 400 mg
(n = 361) |
|
|
HIV-1 RNA <50 copies/mL |
71% |
64% |
|
7.4% (95% CI: 0.7%, 14.2%) |
|
|
Virologic nonresponse |
20% |
28% |
|
No virologic data |
9% |
9% |
|
Reasons | ||
|
3% |
4% |
|
5% |
4% |
|
2% |
1% |
|
Proportion (%) with HIV-1 RNA <50 copies/mL by Baseline Category |
||
|
Plasma viral load (copies/mL) | ||
|
75% |
71% |
|
62% |
47% |
|
Background regimen | ||
|
67% |
60% |
|
85% |
67% |
|
69% |
70% |
|
Gender | ||
|
70% |
66% |
|
74% |
60% |
|
Race | ||
|
75% |
71% |
|
67% |
57% |
a BR = Background regimen. Background regimen was restricted to less than or equal to 2 antiretroviral treatments with at least 1 fully active agent.
b Adjusted for pre-specified stratification factors.
c Other includes reasons such as withdrew consent, loss to follow-up, moved, and protocol deviation.
Treatment differences were maintained across the baseline characteristics including CD4+ cell count and age.
The mean changes in CD4+ cell counts from baseline were 162 cells per mm 3 in the group receiving TIVICAY and 153 cells per mm 3 in the raltegravir group.
Treatment-Experienced, Integrase Strand Transfer Inhibitor-Experienced Subjects
VIKING-3 examined the effect of TIVICAY 50 mg twice daily over 7 days of functional monotherapy, followed by optimized background therapy (OBT) with continued treatment of TIVICAY 50 mg twice daily.
In the multicenter, open-label, single-arm VIKING-3 trial, 183 HIV‑1‑infected, antiretroviral treatment-experienced adults with virological failure and current or historical evidence of raltegravir and/or elvitegravir resistance received TIVICAY 50 mg twice daily with the current failing background regimen for 7 days, then received TIVICAY with OBT from Day 8. A total of 183 subjects enrolled: 133 subjects with INSTI resistance at screening and 50 subjects with only historical evidence of resistance (and not at screening). At baseline, median age of subjects was 48 years; 23% were female, 29% non-white, and 20% had hepatitis B and/or C virus co-infection. Median baseline CD4+ cell count was 140 cells per mm 3, median duration of prior antiretroviral treatment was 13 years, and 56% were CDC Class C. Subjects showed multiple-class antiretroviral treatment resistance at baseline: 79% had greater than or equal to 2 NRTI, 75% greater than or equal to 1 NNRTI, and 71% greater than or equal to 2 PI major substitutions; 62% had non-R5 virus.
Mean reduction from baseline in HIV-1 RNA at Day 8 (primary endpoint) was 1.4 log 10 (95% CI: 1.3 log 10, 1.5 log 10). Response at Week 48 was affected by baseline INSTI substitutions [see Microbiology ( 12.4)] .
After the functional monotherapy phase, subjects had the opportunity to re-optimize their background regimen when possible. Week 48 virologic outcomes for VIKING-3 are shown in Table 16.
|
TIVICAY 50 mg Twice Daily + OBT (n = 183) |
|
|
HIV-1 RNA <50 copies/mL |
63% |
|
Virologic nonresponse |
32% |
|
No virologic data Reasons | |
|
3% |
|
Proportion (%) with HIV-1 RNA <50 copies/mL by Baseline Category |
|
|
Gender | |
|
63% |
|
64% |
|
Race | |
|
63% |
|
64% |
Subjects harboring virus with Q148 and with additional Q148-associated secondary substitutions also had a reduced response at Week 48 in a stepwise fashion [see Microbiology ( 12.4)].
The median change in CD4+ cell count from baseline was 80 cells per mm 3 at Week 48.
Virologically Suppressed Subjects
SWORD-1 and SWORD-2 are identical 148-week, Phase 3, randomized, multicenter, parallel-group, non-inferiority trials. A total of 1,024 adult HIV–1-infected subjects who were on a stable suppressive antiretroviral regimen (containing 2 NRTIs plus either an INSTI, an NNRTI, or a PI) for at least 6 months (HIV-1 RNA less than 50 copies per mL), with no history of treatment failure and no known substitutions associated with resistance to dolutegravir or rilpivirine received treatment in the trials. Subjects were randomized 1:1 to continue their current antiretroviral regimen or be switched to TIVICAY 50 mg plus rilpivirine 25 mg administered once daily. The primary efficacy endpoint for the SWORD trial was the proportion of subjects with plasma HIV-1 RNA less than 50 copies per mL at Week 48. The proportion of subjects with HIV-1 RNA less than 50 copies per mL at Week 48 was 95% for both treatment groups; treatment difference and 95% CI was -0.2% (-3.0%, 2.5%). The proportion of subjects with HIV-1 RNA greater than or equal to 50 copies per mL (virologic failure) at Week 48 was 0.6% and 1.2% for the dolutegravir plus rilpivirine treatment group and the current antiretroviral regimen treatment groups, respectively; treatment difference and 95% CI was -0.6% (-1.7%, 0.6%). Refer to the Prescribing Information for JULUCA (dolutegravir and rilpivirine) tablet for complete virologic outcome information.
14.3 Pediatric Subjects
IMPAACT P1093 is a Phase 1/2, 48-week, multicenter, open-label trial to evaluate the pharmacokinetic parameters, safety, tolerability, and efficacy of TIVICAY in combination treatment regimens in HIV‑1‑infected infants, children, and adolescents. Subjects were stratified by age, enrolling adolescents first (Cohort 1: aged 12 to less than 18 years) and then younger children (Cohort 2A: aged 6 to less than 12 years). All subjects received a weight-based dose of TIVICAY [see Dosage and Administration ( 2.2)] .
These 46 subjects had a mean age of 12 years (range: 6 to 17), were 54% female and 52% black. At baseline, mean plasma HIV-1 RNA was 4.6 log 10 copies per mL, median CD4+ cell count was 639 cells per mm3 (range: 9 to 1,700), and median CD4+% was 23% (range: 1% to 44%). Overall, 39% had baseline plasma HIV-1 RNA greater than 50,000 copies per mL and 33% had a CDC HIV clinical classification of category C. Most subjects had previously used at least 1 NNRTI (50%) or 1 PI (70%).
At Week 24, the proportion of subjects with HIV-1 RNA less than 50 copies per mL in Cohort 1 and Cohort 2A was 70% (16/23) and 61% (14/23), respectively. At Week 48, the proportion of subjects from Cohort 1 with HIV-1 RNA less than 50 copies per mL was 61% (14/23). Virologic outcomes were also evaluated based on body weight. Across both cohorts, virologic suppression (HIV-1 RNA less than 50 copies per mL) at Week 24 was achieved in 75% (18/24) of subjects weighing at least 40 kg and 55% (6/11) of subjects in the 30 to less than 40 kg weight-band. At Week 48, 63% (12/19) of the subjects in Cohort 1 weighing at least 40 kg were virologically suppressed.
The median CD4+ cell count increase from baseline to Week 48 was 84 cells per mm 3 in Cohort 1. For Cohort 2A, the median CD4+ cell count increase from baseline to Week 24 was 209 cells per mm 3.
16 HOW SUPPLIED/STORAGE AND HANDLING
TIVICAY tablets, 10 mg, are white, round, film-coated, biconvex tablets debossed with “SV 572” on one side and “10” on the other side. Bottle of 30 tablets with child-resistant closure and containing a desiccant. NDC 49702-226-13.
Store and dispense the 10-mg tablets in the original package, protect from moisture, and keep the bottle tightly closed. Do not remove desiccant.
TIVICAY tablets, 25 mg, are pale yellow, round, film-coated, biconvex tablets debossed with “SV 572” on one side and “25” on the other side. Bottle of 30 tablets with child-resistant closure. NDC 49702-227-13.
TIVICAY tablets, 50 mg, are yellow, round, film-coated, biconvex tablets debossed with “SV 572” on one side and “50” on the other side. Bottle of 30 tablets with child-resistant closure. NDC 49702-228-13.
Store at 25 °C (77 °F); excursions permitted 15 ° to 30 °C (59 ° to 86 °F) [See USP Controlled Room Temperature].
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Drug Interactions
TIVICAY may interact with other drugs; therefore, advise patients to report to their healthcare provider the use of any other prescription or nonprescription medication or herbal products, including St. John’s wort [see Contraindications ( 4), Drug Interactions ( 7)] .
Hypersensitivity Reactions
Advise patients to immediately contact their healthcare provider if they develop rash. Instruct patients to immediately stop taking TIVICAY and other suspect agents, and seek medical attention if they develop a rash associated with any of the following symptoms, as it may be a sign of a more serious reaction such as severe hypersensitivity: fever; generally ill feeling; extreme tiredness; muscle or joint aches; blisters or peeling of the skin; oral blisters or lesions; eye inflammation; facial swelling; swelling of the eyes, lips, tongue, or mouth; breathing difficulty; and/or signs and symptoms of liver problems (e.g., yellowing of the skin or whites of the eyes, dark or tea-colored urine, pale-colored stools or bowel movements, nausea, vomiting, loss of appetite, or pain, aching, or sensitivity on the right side below the ribs) [see Warnings and Precautions ( 5.1)] .
Hepatotoxicity
Inform patients that hepatotoxicity has been reported with dolutegravir [see Warnings and Precautions (5.2)]. Advise patients that laboratory monitoring for hepatoxicity during therapy with TIVICAY is recommended, especially for patients with liver disease, such as hepatitis B or C.
Immune Reconstitution Syndrome
Advise patients to inform their healthcare provider immediately of any signs or symptoms of infection as inflammation from previous infection may occur soon after combination antiretroviral therapy, including when TIVICAY is started [see Warnings and Precautions (5.3)].
Pregnancy Registry
Advise patients that there is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to TIVICAY during pregnancy [see Use in Specific Populations ( 8.1)] .
Lactation
Instruct women with HIV-1 infection not to breastfeed because HIV-1 can be passed to the baby in the breast milk [see Use in Specific Populations ( 8.2)] .
Missed Dosage
Instruct patients that if they miss a dose of TIVICAY, to take it as soon as they remember. Advise patients not to double their next dose or take more than the prescribed dose [see Dosage and Administration ( 2)] .
Storage
Instruct patients to store the TIVICAY 10-mg tablets in the original package, keep the bottle tightly closed, and protect from moisture. Do not remove desiccant [see How Supplied/Storage and Handling ( 16)] .
TIVICAY, EPZICOM, JULUCA, and TRIUMEQ are trademarks owned by or licensed to the ViiV Healthcare group of companies.
The other brands listed are trademarks owned by or licensed to their respective owners and are not owned by or licensed to the ViiV Healthcare group of companies. The makers of these brands are not affiliated with and do not endorse the ViiV Healthcare group of companies or its products.
Manufactured for:
ViiV Healthcare
Research Triangle Park, NC 27709
by:
GlaxoSmithKline
Research Triangle Park, NC 27709
©2017 ViiV Healthcare group of companies or its licensor.
TVC:9PI
PHARMACIST‑DETACH HERE AND GIVE INSTRUCTIONS TO PATIENT
_ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _
|
PATIENT INFORMATION |
|||||
|
TIVICAY (TIV-eh-kay) (dolutegravir) tablets |
|||||
|
What is TIVICAY? TIVICAY is a prescription medicine that is used to treat Human Immunodeficiency Virus-1 (HIV-1) infection together with:
HIV-1 is the virus that causes Acquired Immune Deficiency Syndrome (AIDS). It is not known if TIVICAY is safe and effective in children who weigh less than 66 pounds (30 kg) or in children who have received certain types of medicine for HIV-1 infection. |
|||||
|
Do not take TIVICAY if you:
|
|||||
|
Before you take TIVICAY, tell your healthcare provider about all of your medical conditions, including if you:
Pregnancy Registry. There is a pregnancy registry for women who take antiretroviral medications, including TIVICAY during pregnancy. The purpose of this registry is to collect information about the health of you and your baby. Talk to your healthcare provider about how you can take part in this registry.
Talk with your healthcare provider about the best way to feed your baby. Tell your healthcare provider about the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Some medicines interact with TIVICAY. Keep a list of your medicines and show it to your healthcare provider and pharmacist when you get a new medicine.
|
|||||
|
How should I take TIVICAY?
|
|||||
|
What are the possible side effects of TIVICAY?
|
|||||
|
|
||||
|
|||||
|
|
||||
|
|||||
|
|
|
|||
|
These are not all the possible side effects of TIVICAY. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1‑800‑FDA‑1088. |
|||||
|
How should I store TIVICAY?
Keep TIVICAY and all medicines out of the reach of children. |
|||||
|
General information about the safe and effective use of TIVICAY. Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use TIVICAY for a condition for which it was not prescribed. Do not give TIVICAY to other people, even if they have the same symptoms you have. It may harm them. You can ask your healthcare provider or pharmacist for information about TIVICAY that is written for health professionals. For more information, go to www.TIVICAY.com or call 1-877-844-8872. |
|||||
|
What are the ingredients in TIVICAY? Active ingredient: dolutegravir. Inactive ingredients: D-mannitol, microcrystalline cellulose, povidone K29/32, sodium starch glycolate, and sodium stearyl fumarate. The tablet film‑coating contains the inactive ingredients iron oxide yellow (for the 25-mg and 50-mg tablets only), macrogol/PEG, polyvinyl alcohol-part hydrolyzed, talc, and titanium dioxide. Manufactured for: by: ViiV Healthcare GlaxoSmithKline Research Triangle Park, NC 27709 Research Triangle Park, NC 27709 Trademark is owned by or licensed to the ViiV Healthcare group of companies. ©2017 ViiV Healthcare group of companies or its licensor. TVC:7PIL |
|||||
|
This Patient Information has been approved by the U.S. Food and Drug Administration. |
Revised: 11/2017 |
||||
DRUG: Tivicay
GENERIC: dolutegravir sodium
DOSAGE: TABLET, FILM COATED
ADMINSTRATION: ORAL
NDC: 61786-771-02
COLOR: yellow
SHAPE: ROUND
SCORE: No score
SIZE: 9 mm
IMPRINT: SV;572;50
PACKAGING: 30 in 1 BLISTER PACK
ACTIVE INGREDIENT(S):
- DOLUTEGRAVIR SODIUM 50mg in 1
INACTIVE INGREDIENT(S):
- FERRIC OXIDE YELLOW
- POLYETHYLENE GLYCOL, UNSPECIFIED
- MANNITOL
- POLYVINYL ALCOHOL, UNSPECIFIED
- TALC
- MICROCRYSTALLINE CELLULOSE
- POVIDONE, UNSPECIFIED
- SODIUM STEARYL FUMARATE
- SODIUM STARCH GLYCOLATE TYPE A CORN
- TITANIUM DIOXIDE

| TIVICAY
dolutegravir sodium tablet, film coated |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Labeler - REMEDYREPACK INC. (829572556) |