SOOTHE LONG LASTING- povidone solution
Bausch & Lomb Incorporated
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
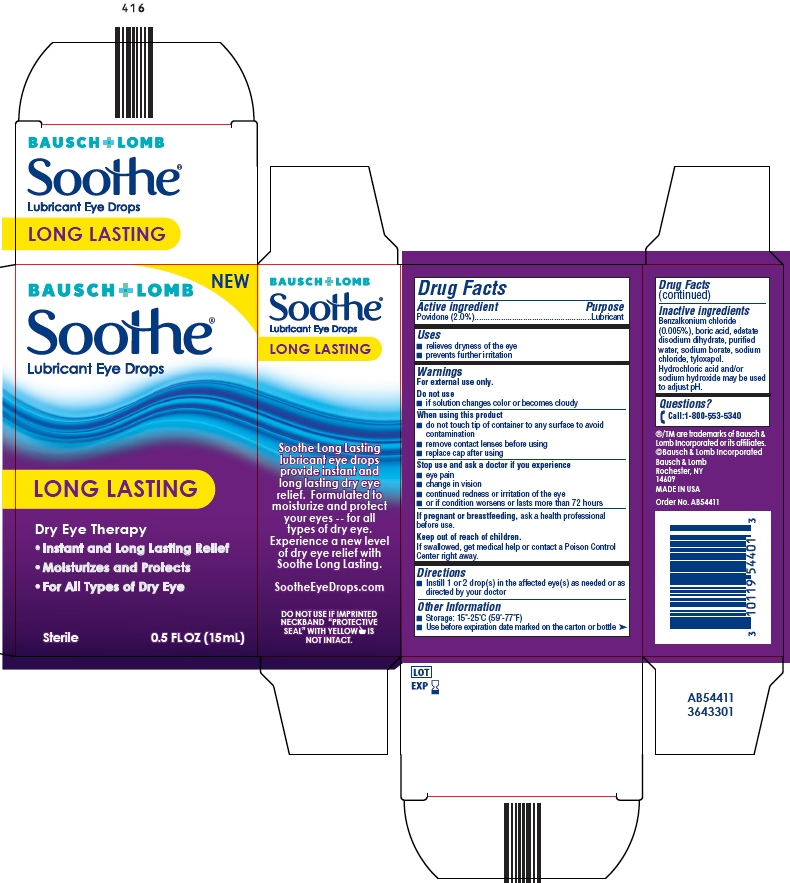
Drug Facts
Warnings
For external use only.
When using this product
■ do not touch tip of container to any surface to avoid contamination
■ remove contact lenses before using
■ replace cap after using
Keep out of reach of children
If swallowed, get medical help or contact a Poison Control
Center right away.
Other information
■ Storage: 15°-25°C (59°-77°F)
■ Use before expiration date marked on the carton or bottle
| SOOTHE LONG LASTING
povidone solution |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Labeler - Bausch & Lomb Incorporated (196603781) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Bausch & Lomb Incorporated | 079587625 | MANUFACTURE(24208-544) | |
Revised: 12/2017
Document Id: c6463480-cd30-4020-ab7b-d7e361997930
Set id: 30a388e9-b7e7-400d-8574-baf60a59375f
Version: 3
Effective Time: 20171220
Bausch & Lomb Incorporated