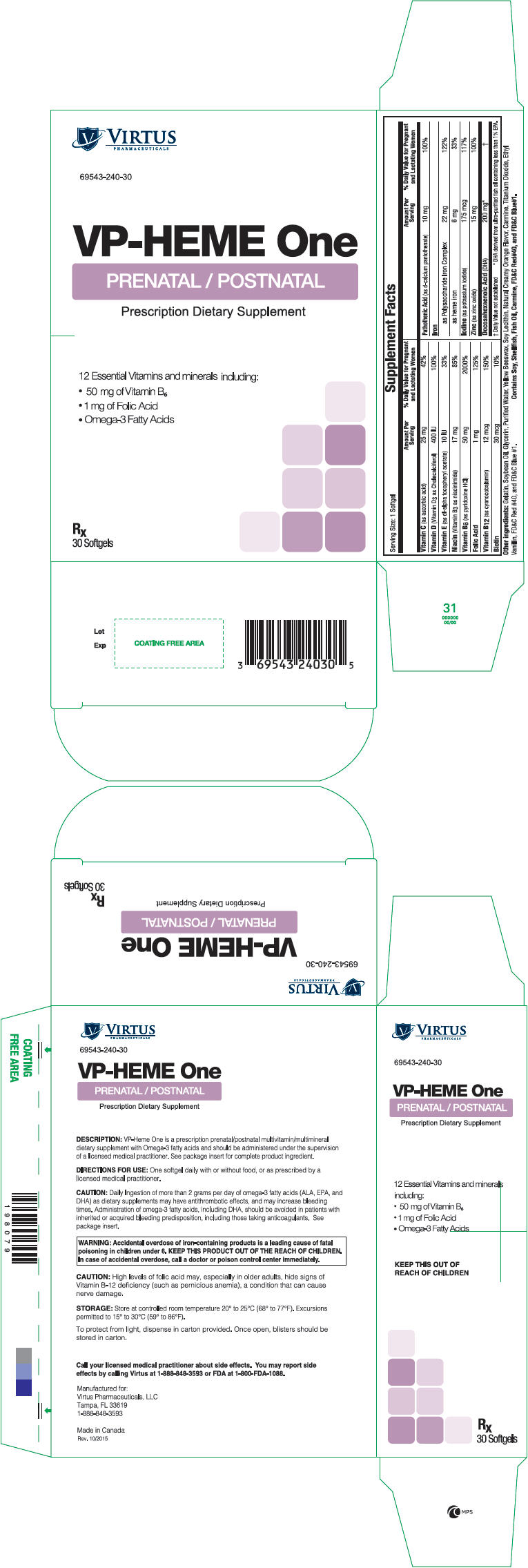
VP-HEME ONE- ascorbic acid, cholecalciferol, .alpha.-tocopherol acetate, niacinamide, pyridoxine hydrochloride, folic acid, cyanocobalamin, biotin, calcium pantothenate, iron sucrose, heme iron polypeptide, potassium iodide, zinc oxide, and doconexent capsule, gelatin coated
Virtus Pharmaceuticals
----------
VP-HEME One
DESCRIPTION
VP-HEME One is a prescription prenatal/postnatal multivitamin/multimineral dietary supplement with Omega-3 fatty acids.
DIRECTIONS FOR USE
Before, during, and/or after pregnancy, one softgel daily with or without food, or as prescribed by a licensed medical practitioner.
| Supplement Facts
Serving Size: 1 Softgel |
||
|---|---|---|
| Amount
Per Serving | % Daily Value for Pregnant & Lactating Women | |
|
Vitamin C (as ascorbic acid) |
25 mg |
42% |
|
Vitamin D (Vitamin D3 as cholecalciferol) |
400 IU |
100% |
|
Vitamin E (as dl-alpha tocopheryl acetate) |
10 IU |
33% |
|
Niacin (Vitamin B3 as niacinimide) |
17 mg |
85% |
|
Vitamin B6 (as pyridoxine HCl) |
50 mg |
2000% |
|
Folic Acid |
1 mg |
125% |
|
Vitamin B12 (as cyanocobalamin) |
12 mcg |
150% |
|
Biotin |
30 mcg |
10% |
|
Pantothenic Acid
|
10 mg |
100% |
|
Iron | ||
|
as Polysaccharide Iron Complex |
22 mg |
122% |
|
as heme iron |
6 mg |
33% |
|
Iodine (as potassium iodide) |
175 mcg |
117% |
|
Zinc (as zinc oxide) |
15 mg |
100% |
|
Docosahexaenoic Acid (DHA) |
200 mg* | |
Other Ingredients: Gelatin, Soybean Oil, Glycerin, Purified Water, Yellow Beeswax, Soy Lecithin, Natural Creamy Orange Flavor, Carmine, Titanium Dioxide, Ethyl Vanillin, FD&C Red #40, and FD&C Blue #1.
Contains Soy, Shellfish, Fish Oil, Carmine, FD&C Red #40, and FD&C Blue #1.
ALLERGY POTENTIAL
This product has been manufactured in a facility that also manufactures products containing tree nuts, peanuts, fish, egg, wheat, milk, soy and shellfish. Individuals with allergic tendencies to these substances should use discretion.
KEEP THIS PRODUCT OUT OF THE REACH OF CHILDREN.
CONTRAINDICATIONS
This product is contraindicated in patients with a known hypersensitivity to any of the ingredients.
|
WARNING: Accidental overdose of iron-containing products is a leading cause of fatal poisoning in children under 6. KEEP THIS PRODUCT OUT OF REACH OF CHILDREN. In case of accidental overdose, call a doctor or poison control center immediately. |
CAUTIONS
High levels of folic acid may, especially in older adults, hide signs of Vitamin B-12 deficiency (such as pernicious anemia), a condition that can cause nerve damage.
Ingestion of more than 2 grams per day of omega-3 fatty acids (ALA, EPA, and DHA) as dietary supplements may have antithrombotic effects and may increase bleeding times. Administration of omega-3 fatty acids, including DHA, should be avoided in patients with inherited or acquired bleeding predisposition, including those taking anticoagulants.
SIDE EFFECTS
Allergic sensitization has been reported following oral administration of folic acid.
Paresthesia, somnolence, nausea and headaches have been reported with pyridoxine hydrochloride (Vitamin B6).
Mild transient diarrhea, polycythemia vera, itching, transitory exanthema and the feeling of swelling of the entire body have been associated with vitamin B12.
Iron can cause mild gastrointestinal side effects, particularly when taken on an empty stomach.
Omega-3 fatty acids are associated with side effects in fewer than 7% of persons who consume them; if they occur, side effects are usually mild gastrointestinal upsets.1
Call your medical practitioner about side effects.
DRUG INTERACTIONS
Vitamin B6 (Pyridoxine hydrochloride) should not be given to patients receiving the drug levodopa, because the action of levodopa is antagonized by pyridoxine hydrochloride. However, pyridoxine hydrochloride may be used concurrently in patients receiving a preparation containing both carbidopa and levodopa.
High doses of Vitamin E have been associated with the risk of hemorrhage in patients receiving anticoagulants, mineral oil and orlistat may impair absorption of Vitamin E, and Vitamin E can potentially increase absorption, utilization and storage of Vitamin A.
Drugs which may interact with folate include:
- •
- Antiepileptic drugs (AED): The AED class including, but not limited to, phenytoin, carbamazepine, primidone, valproic acid, fosphenytoin, valproate, phenobarbital and lamotrigine have been shown to impair folate absorption and increase the metabolism of circulating folate. Additionally, concurrent use of folic acid has been associated with enhanced phenytoin metabolism, lowering the level of the AED in the blood and allowing breakthrough seizures to occur. Caution should be used when prescribing this product among patients who are receiving treatment with phenytoin or other anticonvulsants.
- •
- Capecitabine: Folinic acid (5-formyltetrahydrofolate) may increase the toxicity of Capecitabine.
- •
- Cholestyramine: Reduces folic acid absorption and reduces serum folate levels.
- •
- Colestipol: Reduces folic acid absorption and reduces serum folate levels.
- •
- Cycloserine: Reduces folic acid absorption and reduces serum folate levels.
- •
- Dihydrofolate Reductase Inhibitors (DHFRI): DHFRIs block the conversion of folic acid to its active forms, and lower plasma and red blood cell folate levels. DHFRIs include aminopterin, methotrexate, pyrimethamine, triamterene, and trimethoprim. Caution should be exercised when using folate with folate antagonists. Patients, typically, should not be given folate simultaneously with a folate antagonist, for the purpose of reducing or preventing clinical toxicity, as the therapeutic effect of the antagonist may be nullified.
- •
- Fluoxetine: Fluoxetine exerts a noncompetitive inhibition of the 5-methyltetrahydrofolate active transport in the intestine.
- •
- Isotretinoin: Reduced folate levels have occurred in some patients taking isotretinoin.
- •
- L-dopa, triamterene, colchicine, and trimethoprim may decrease plasma folate levels.
- •
- Nonsteroidal Anti-inflammatory Drugs (NSAIDs): NSAIDs have been shown to inhibit some folate dependent enzymes in laboratory experiments. NSAIDs include ibuprofen, naproxen, indomethacin and sulindac.
- •
- Oral Contraceptives: Serum folate levels may be depressed by oral contraceptive therapy.
- •
- Methylprednisolone: Reduced serum folate levels have been noted after treatment with methylprednisolone.
- •
- Pancreatic Enzymes, including, but not limited to pancreatin and pancreplipase: Reduced folate levels have occurred in some patients taking pancreatic extracts.
- •
- Pentamidine: Reduced folate levels have been seen with prolonged intravenous pentamidine.
- •
- Pyrimethamine: High levels of folic acid may result in decreased serum levels of pyrimethamine.
- •
- Smoking and Alcohol: Reduced serum folate levels have been noted.
- •
- Sulfasalazine: Inhibits the absorption and metabolism of folic acid.
- •
- Warfarin can produce significant impairment in folate status after a 6-month therapy.
- •
- Heme-iron: Can compete for transport and reduce folate absorption. Ensure adequate medical supervision to ensure proper iron levels.
- •
- Folinic acid may enhance the toxicity of fluorouracil.
- •
- Concurrent administration of chloramphenicol and folate in folate-deficient patients may result in antagonism of the hematopoietic response to folate.
- •
- Caution should be exercised with the concomitant use of folate and trimethoprim-sulfamethoxazole for the acute treatment of Pneumocystis carinii pneumonia in patients with HIV infection as it is associated with increased rates of treatment failure and mortality in a placebo controlled study.
Drugs which may interact with Vitamin B6 include:
- •
- Vitamin B6 should not be given to patients receiving the drug levodopa because the action of levodopa is antagonized by vitamin B6. However, Vitamin B6 may be used concurrently in patients receiving a preparation containing both carbidopa and levodopa.
- •
- Isoniazid can produce a Vitamin B6 deficiency.
Drug interactions with Vitamin B12 include:
- •
- Antibiotics, cholestyramine, colchicines, colestipol, metformin, para-aminosalicylic acid, and potassium chloride may decrease the absorption of Vitamin B12.
- •
- Nitrous oxide can produce a functional Vitamin B12 deficiency.
Drug interactions with Vitamin D include:
- •
- Certain thiazide diuretics, such as hydrochlorothiazide, as well as antacids, bile acid sequestrants (such as cholestyramine), mineral oil, orlistat, olestra, cimetidine, and anticonvulsant medications may reduce the absorption or increase the catabolism of Vitamin D.
- •
- Vitamin D supplementation should not be given with calcium in those with hypercalcemia or conditions that may lead to hypercalcemia such as hyperparathyroidism and those who form calcium-containing kidney stones.
Drug interactions with Iron include:
- 1.
- Iron supplements might reduce the amounts of levodopa available to the body and diminish its clinical effectiveness.
- 2.
- Levothyroxine ingested simultaneously with iron can result in clinically significant reductions in levothyroxine efficacy.
- 3.
- Proton pump inhibitors reduce the acidity of stomach contents and can reduce iron absorption.
Drugs that interact with Iodine:
- 1.
- Anti-thyroid medications, such as methimazole, are used to treat hyperthyroidism. Taking high doses of iodine can produce an additive effect and cause hypothyroidism.
- 2.
- Taking potassium iodide with angiotensin-converting enzyme (ACE) inhibitors increases the risk of hyperkalemia.
- 3.
- Potassium-sparing diuretics such as spironolactone and amiloride can increase the risk of hyperkalemia when taken with potassium iodide.
Drugs that interact with Zinc:
- 1.
- Quinolone and tetracycline antibiotics interact with zinc in the gastrointestinal tract, inhibiting the absorption of both zinc and the antibiotic.
- 2.
- The absorption and action of penicillamine can be reduced by zinc.
- 3.
- Thiazide diuretics increase urinary zinc excretion.
- 4.
- Iron supplementation might decrease zinc absorption.
- 5.
- High zinc intake can inhibit copper absorption.
HEALTH CLAIM
Adequate folate in healthful diets may reduce a woman's risk of having a child with a brain or spinal cord birth defect.
HOW SUPPLIED
Supplied as dark purple, oblong softgel, imprinted "240" dispensed in boxes of 30 softgels (10 × 3 blister packs).
STORAGE
Store at controlled room temperature 20° to 25°C (68° to 77°F). Excursions permitted to 15° to 30°C (59° to 86°F). To protect from light, dispense in carton provided. Once open, blisters should be stored in carton.
KEEP OUT OF REACH OF CHILDREN.
Call your licensed medical practitioner about side effects. You may report side effects by calling Virtus at 1-888-848-3593 or FDA at 1-800-FDA-1088.
REFERENCES
- 1.
- Omega-3 Fatty Acids and Health: Fact Sheet for Health Professionals. National Institutes of Health. 2005. http://ods.od.nih.gov/factsheets/Omega3FattyAcidsand Health-HealthProfessional/. Accessed on 10/12/2015.
- 2.
- U.S. Food and Drug Administration (2013). "Guidance for Industry: A Food Labeling Guide (12. Appendix D: Qualified Health Claims). http://www.fda.gov/Food/GuidanceRegulation/ GuidanceDocumentsRegulatory Information/Labeling Nutrition/ucm064923.htm. Accessed on 10/12/2015.
| VP-HEME ONE
ascorbic acid, cholecalciferol, .alpha.-tocopherol acetate, niacinamide, pyridoxine hydrochloride, folic acid, cyanocobalamin, biotin, calcium pantothenate, iron sucrose, heme iron polypeptide, potassium iodide, zinc oxide, and doconexent capsule, gelatin coated |
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
| Supplement Facts | ||
| Serving Size : | Serving per Container : | |
| Amount Per Serving | % Daily Value | |
|---|---|---|
| color | ||
| size (solid drugs) | 21 mm | |
| shape | ||
| scoring | 1 | |
| imprint | ||
| flavor | ||
| Labeler - Virtus Pharmaceuticals (079659493) |