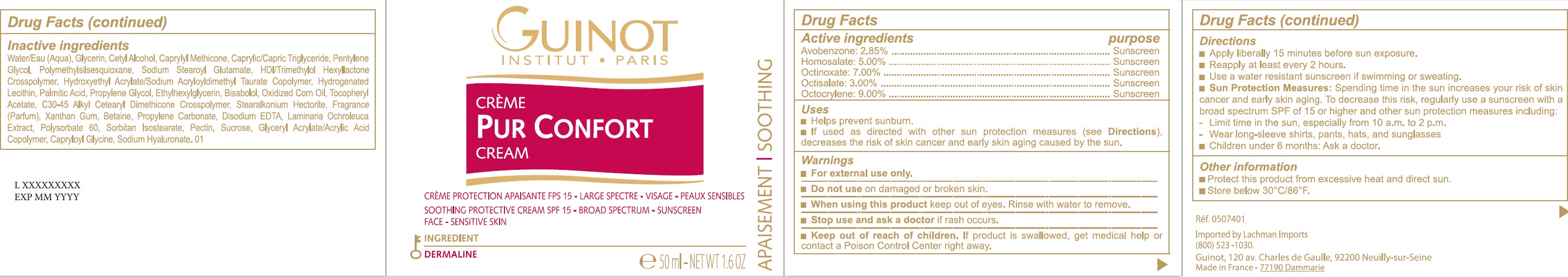
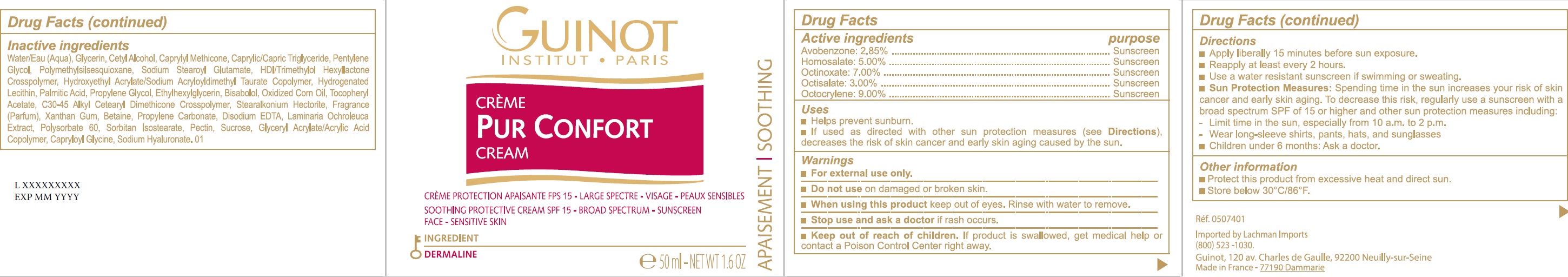
Label: PUR CONFORT SPF 15- avobenzone, homosalate, octinoxate, octisalate, octocrylene cream
- NDC Code(s): 54181-015-50
- Packager: Guinot
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 19, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredients
- Uses
- Warnings
-
Directions
- Apply liberally 15 minutes before sun exposure.
- Reapply at least every 2 hours.
- Use a water resistant sunscreen if swimming or sweating.
- Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a broad spectrum SPF of 15 or higher and other sun protection measures including: Sun Protection Measures:
- Limit time in the sun, especially from 10 a.m. to 2 p.m.
- Wear long-sleeve shirts, pants, hats, and sunglasses
- Children under 6 months: Ask a doctor.
- Other information
-
Inactive ingredients
Water/Eau (Aqua), Glycerin, Cetyl Alcohol, Caprylyl Methicone, Caprylic/Capric Triglyceride, Pentylene Glycol, Polymethylsilsesquioxane, Sodium Stearoyl Glutamate, HDI/Trimethylol Hexyllactone Crosspolymer, Hydroxyethyl Acrylate/Sodium Acryloyldimethyl Taurate Copolymer, Hydrogenated Lecithin, Palmitic Acid, Propylene Glycol, Ethylhexylglycerin, Bisabolol, Oxidized Corn Oil, Tocopheryl Acetate, C30-45 Alkyl Cetearyl Dimethicone Crosspolymer, Stearalkonium Hectorite, Fragrance (Parfum), Xanthan Gum, Betaine, Propylene Carbonate, Disodium EDTA, Laminaria Ochroleuca Extract, Polysorbate 60, Sorbitan Isostearate, Pectin, Sucrose, Glyceryl Acrylate/Acrylic Acid Copolymer, Capryloyl Glycine, Sodium Hyaluronate. 01
- Package Labeling
-
INGREDIENTS AND APPEARANCE
PUR CONFORT SPF 15
avobenzone, homosalate, octinoxate, octisalate, octocrylene creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:54181-015 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 28.5 mg in 1 mL HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 50 mg in 1 mL OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 70 mg in 1 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 30 mg in 1 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 90 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) CETYL ALCOHOL (UNII: 936JST6JCN) CAPRYLYL TRISILOXANE (UNII: Q95M2P1KJL) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) PENTYLENE GLYCOL (UNII: 50C1307PZG) POLYMETHYLSILSESQUIOXANE (4.5 MICRONS) (UNII: 59Z907ZB69) SODIUM STEAROYL GLUTAMATE (UNII: 65A9F4P024) HEXAMETHYLENE DIISOCYANATE/TRIMETHYLOL HEXYLLACTONE CROSSPOLYMER (UNII: WB5K9Y35Y9) HYDROXYETHYL ACRYLATE/SODIUM ACRYLOYLDIMETHYL TAURATE COPOLYMER (100000 MPA.S AT 1.5%) (UNII: 86FQE96TZ4) HYDROGENATED SOYBEAN LECITHIN (UNII: H1109Z9J4N) PALMITIC ACID (UNII: 2V16EO95H1) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) LEVOMENOL (UNII: 24WE03BX2T) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) STEARALKONIUM HECTORITE (UNII: OLX698AH5P) XANTHAN GUM (UNII: TTV12P4NEE) BETAINE (UNII: 3SCV180C9W) PROPYLENE CARBONATE (UNII: 8D08K3S51E) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) LAMINARIA OCHROLEUCA (UNII: 4R2124HE76) POLYSORBATE 60 (UNII: CAL22UVI4M) SORBITAN ISOSTEARATE (UNII: 01S2G2C1E4) PECTIN (UNII: 89NA02M4RX) SUCROSE (UNII: C151H8M554) CAPRYLOYL GLYCINE (UNII: 8TY5YO42NJ) HYALURONATE SODIUM (UNII: YSE9PPT4TH) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54181-015-50 1 in 1 BOX 05/01/2018 1 50 mL in 1 JAR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 05/01/2018 Labeler - Guinot (763667185)