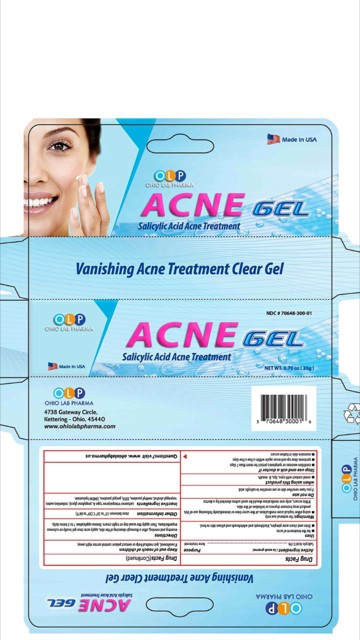
OLP ACNE CLEAR- salicylic acid gel
OHIO LAB PHARMA
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
USES
For the treatment of acne. Dries and clears acne pimples, blackheads and whiteheads and allows skin to heal.
DIRECTIONS
Morning and evening, after a thorough cleansing of the skin, apply acne clear gel locally on cutanous imperfections. Then apply the usual day or night cream. Renew application 1 to 3 times daily.
WARNINGS
For external use only. Using other topical acne medications at the same time or immediately following use of this product may increase dryness or irritation of the skin. If this occurs, only one medication should be used unless directed by a doctor. Avoid direct contact with the eyes. If product gets into the eyes, rinse liberally with water. Discontinue use if skin irritation develops or increases. If irritation persists, consult a doctor.
KEEP OUT OF REACH OF CHILDREN
if swallowed, get medical help or contact poison control center right away
| OLP ACNE CLEAR
salicylic acid gel |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - OHIO LAB PHARMA (080215854) |
| Registrant - OHIO LAB PHARMA (080215854) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| OHIO LAB PHARMA | 080215854 | manufacture(70648-300) | |