SL EYE WASH WITH PRESERVATIVE- water irrigant
Aaxis Pacific dba Aaxis Pharmaceuticals
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
SL Eye Wash
with Preservative
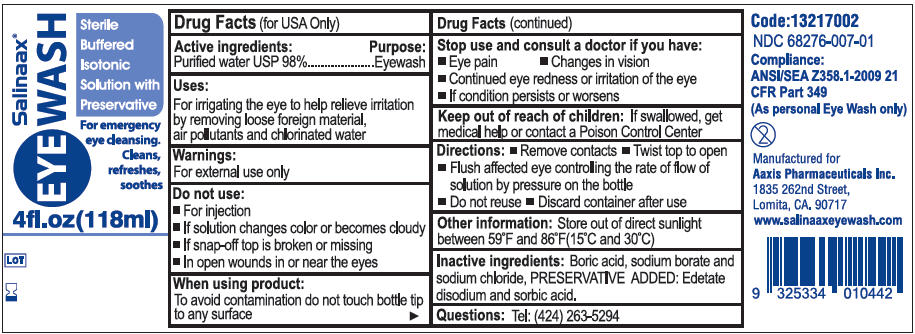
Uses
For irrigating the eye to help relieve irritation by removing loose foreign material, air pollutants and chlorinated water
Warnings
For external use only
Do not use
- If solution changes color or becomes cloudy
- If snap-off top is broken or missing
- For injection
- In open wounds in or near the eyes
Directions
- Remove contacts
- Twist top to open
- Flush affected eye controlling the rate of flow of solution by pressure on the bottle
- Do not reuse
- Discard container after use
| SL EYE WASH
WITH PRESERVATIVE
water irrigant |
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Aaxis Pacific dba Aaxis Pharmaceuticals (049082677) |
Revised: 7/2021
Document Id: c704d791-a40d-92fb-e053-2a95a90ac14b
Set id: 2f8113ca-acca-46a6-93b0-643a080c8a84
Version: 3
Effective Time: 20210713
Aaxis Pacific dba Aaxis Pharmaceuticals