Label: UREA- urea cream
- NDC Code(s): 54295-311-18
- Packager: Trinity Pharmaceuticals, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated March 22, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
Rx Only
For external use only. Not for ophthalmic use.
Description
Urea Cream 39% is a keratolytic emollient which is a gentle, yet potent, tissue softener for nails and/or skin. Each gram of Urea Cream 39% contains 39% urea as an active ingredient, and the following inactive ingredients: Water, Propylene Glycol, Glyceryl Stearate, Mineral Oil, Cetyl Alcohol, Carbomer, Petrolatum, Xanthan Gum and Sodium Hydroxide.
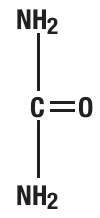
Urea is a diamide of carbonic acid with the following chemical structure:
- Clinical Pharmacology
- Pharmacokinetics
-
Indications and Usage
For debridement and promotion of normal healing of hyperkeratotic surface lesions, particularly where healing is retarded by local infection, necrotic tissue, fibrinous or purulent debris or eschar. Urea is useful for the treatment of hyperkeratotic conditions such as dry, rough skin, dermatitis, psoriasis, xerosis, ichthyosis, eczema, keratosis pilaris, keratosis palmaris, keratoderma, corns and calluses, as well as damaged, ingrown and devitalized nails.
- Contraindications
- Warnings
- Precautions
-
PREGNANCY
PREGNANCY: Pregnancy Category B. Animal reproduction studies have revealed no evidence of harm to the fetus, however, there are no adequate and well-controlled studies in pregnant women. Because animal reproductive studies are not always predictive of human response, Urea Cream 39% should be given to a pregnant woman only if clearly needed.
- NURSING MOTHERS
- Adverse Reactions
- Dosage and Administration
-
How Supplied
Urea 39% Cream 8 oz. (226.8g): NDC 54295-311-18
Store at room temperature 15°C - 30°C (59°F-86°F). Protect from freezing. Keep bottle tightly closed.
KEEP THIS AND ALL MEDICATIONS OUT OF THE REACH OF CHILDREN.
Marketed by:
Trinity Pharma LLC
2255 Glades Road
Suite 324A
Boca Raton, FL 33431
TrinityPharmaLLC.com - PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
UREA
urea creamProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:54295-311 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength UREA (UNII: 8W8T17847W) (UREA - UNII:8W8T17847W) UREA 39 g in 100 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) MINERAL OIL (UNII: T5L8T28FGP) CETYL ALCOHOL (UNII: 936JST6JCN) CARBOMER HOMOPOLYMER TYPE A (ALLYL PENTAERYTHRITOL CROSSLINKED) (UNII: F68VH75CJC) PETROLATUM (UNII: 4T6H12BN9U) XANTHAN GUM (UNII: TTV12P4NEE) SODIUM HYDROXIDE (UNII: 55X04QC32I) Product Characteristics Color white Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54295-311-18 1 in 1 CARTON 12/02/2013 1 227 g in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 12/02/2013 Labeler - Trinity Pharmaceuticals, LLC (078671698)


