ANALPRAM ADVANCED- hydrocortisone acetate and pramoxine hydrochloride
Sebela Pharmaceuticals Inc.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
Analpram HC ® (hydrocortisone acetate 2.5% pramoxine HCl 1%) Cream 2.5%
DESCRIPTION: Analpram HC
® Cream 2.5%
is a topical preparation containing hydrocortisone acetate 2.5% w/w and pramoxine hydrochloride 1% w/w in a hydrolipid base containing stearic acid, cetyl alcohol, Aquaphor®, isopropyl palmitate, polyoxyl 40 stearate, propylene glycol, potassium sorbate, sorbic acid, triethanolamine lauryl sulfate, and purified water.
Topical corticosteroids are anti-inflammatory and anti-pruritic agents. The structural formula, chemical name, molecular formula and molecular weight for active ingredients are presented below.
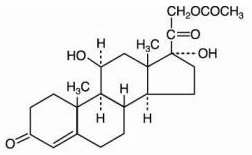
hydrocortisone acetate
Pregn-4-ene-3,20-dione, 21-(acetyloxy)-11, 17-dihydroxy-, (11-beta)-
C
23H
32O
6; mol. wt.: 404.50
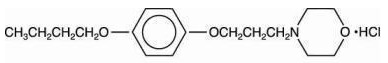
pramoxine hydrochloride
4-(3-(p-butoxyphenoxy)propyl)morpholine hydrochloride
C
17H
27NO
3.HCl; mol. wt.: 329.87
CLINICAL PHARMACOLOGY: Topical corticosteroids share anti-inflammatory, anti-pruritic and vasoconstrictive actions.
The mechanism of anti-inflammatory activity of topical corticosteroids is unclear. Various laboratory methods, including vasoconstrictor assays, are used to compare and predict potencies and/or clinical efficacies of the topical corticosteroids. There is some evidence to suggest that a recognizable correlation exists between vasoconstrictor potency and therapeutic efficacy in man.
Pramoxine hydrochloride is a topical anesthetic agent which provides temporary relief from itching and pain. It acts by stabilizing the neuronal membrane of nerve endings with which it comes into contact.
Pharmacokinetics: The extent of percutaneous absorption of topical corticosteroids is determined by many factors including the vehicle, the integrity of the epidermal barrier, and the use of occlusive dressings.
Topical corticosteroids can be absorbed from normal intact skin. Inflammation and/or other disease processes in the skin increase percutaneous absorption. Occlusive dressings substantially increase the percutaneous absorption of topical corticosteroids. Thus, occlusive dressings may be a valuable therapeutic adjunct for treatment of resistant dermatoses. (See
DOSAGE AND ADMINISTRATION.)
Once absorbed through the skin, topical corticosteroids are handled through pharmacokinetic pathways similar to systemically administered corticosteroids. Corticosteroids are bound to plasma proteins in varying degrees. Corticosteroids are metabolized primarily in the liver and are then excreted by the kidneys. Some of the topical corticosteroids and their metabolites are also excreted into the bile.
INDICATIONS AND USAGE: Topical corticosteroids are indicated for the relief of the inflammatory and pruritic manifestations of corticosteroid-responsive dermatoses.
CONTRAINDICATIONS: Topical corticosteroids are contraindicated in those patients with a history of hypersensitivity to any of the components of the preparation.
PRECAUTIONS: General: Systemic absorption of topical corticosteroids has produced reversible hypothalamic-pituitary-adrenal (HPA) axis suppression, manifestations of Cushing's syndrome, hyperglycemia, and glucosuria in some patients. Conditions which augment systemic absorption include the application of the more potent steroids, use over large surface areas, prolonged use, and the addition of occlusive dressings.
Therefore, patients receiving a large dose of a potent topical steroid applied to a large surface area and under an occlusive dressing should be evaluated periodically for evidence of HPA axis suppression by using the urinary free Cortisol and ACTH stimulation tests. If HPA axis suppression is noted, an attempt should be made to withdraw the drug, to reduce the frequency of application, or to substitute a less potent steroid.
Recovery of HPA axis function is generally prompt and complete upon discontinuation of the drug. Infrequently, signs and symptoms of steroid withdrawal may occur, requiring supplemental systemic corticosteroids. Children may absorb proportionally larger amounts of topical corticosteroids and thus be more susceptible to systemic toxicity. (See
Precautions-Pediatric Use.)
If irritation develops, topical corticosteroids should be discontinued and appropriate therapy instituted.
In the presence of dermatological infections, the use of an appropriate antifungal or antibacterial agent should be instituted. If a favorable response does not occur promptly the corticosteroid should be discontinued until the infection has been adequately controlled.
Information for the Patient: Patients using topical corticosteroids should receive the following information and instructions:
- This medication is to be used as directed by the physician. It is for external use only. Avoid contact with the eyes.
- Patients should be advised not to use this medication for any disorder other than for which it was prescribed.
- The treated skin area should not be bandaged or otherwise covered or wrapped as to be occlusive unless directed by the physician.
- Patients should report any signs of local adverse reactions especially under occlusive dressings.
- Parents of pediatric patients should be advised not to use tight-fitting diapers or plastic pants on a child being treated in the diaper area, as these garments may constitute occlusive dressings.
Laboratory Tests: The following tests may be helpful in evaluating the HPA axis suppression:
Urinary free Cortisol test
ACTH stimulation test
Carcinogenesis, Mutagenesis, and Impairment of Fertility: Long-term animal studies have not been performed to evaluate the carcinogenic potential or the effect on fertility of topical corticosteroids. Studies to determine mutagenicity with prednisolone and hydro-cortisone have revealed negative results.
Pregnancy: Teratogenic Effects: Pregnancy Category C: Corticosteroids are generally teratogenic in laboratory animals when administered systemically at relatively low dosage levels. The more potent corticosteroids have been shown to be teratogenic after dermal application in laboratory animals. There are no adequate and well-controlled studies in pregnant women on teratogenic effects from topically applied corticosteroids. Therefore, topical corticosteroids should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. Drugs of this class should not be used extensively on pregnant patients, in large amounts, or for prolonged periods of time.
Nursing Mothers: It is not known whether topical administration of corticosteroids could result in sufficient systemic absorption to produce detectable amounts in breast milk.
Systemically administered corticosteroids are secreted into breast milk in quantities NOT likely to have a deleterious effect on the infant. Nevertheless, caution should be exercised when topical corticosteroids are administered to a nursing woman.
Pediatric Use: Pediatric patients may demonstrate greater susceptibility to topical corticosteroid induced HPA axis suppression and Cushing's syndrome than mature patients because of a larger skin surface area to body weight ratio.
Hypothalamic-pituitary-adrenal (HPA) axis suppression, Cushing's syndrome, and intra-cranial hypertension have been reported in children receiving topical corticosteroids. Manifestations of adrenal suppression in children include linear growth retardation, delayed weight gain, low plasma Cortisol levels, and absence of response to ACTH stimulation. Manifestations of intracranial hypertension include bulging fontanelles, headaches, and bilateral papilledema.
Administration of topical corticosteroids to children should be limited to the least amount compatible with an effective therapeutic regimen. Chronic corticosteroid therapy may interfere with the growth and development of children.
ADVERSE REACTIONS: The following local adverse reactions are reported infrequently with topical corticosteroids, but may occur more frequently with the use of occlusive dressings. These reactions are listed in an approximate decreasing order of occurrence:
| Burning | Hypertrichosis | Maceration of the skin |
| Itching | Acneiform eruptions | Secondary infection |
| Irritation | Hypopigmentation | Skin atrophy |
| Dryness | Perioral dermatitis | Striae |
| Folliculitis | Allergic contact dermatitis | Miliaria |
OVERDOSAGE: Topically applied corticosteroids can be absorbed in sufficient amounts to produce systemic effects. (See
PRECAUTIONS.)
DOSAGE AND ADMINISTRATION: Topical corticosteroids are generally applied to the affected area as a thin film three to four times daily depending on the severity of the condition. Occlusive dressings may be used for the management of psoriasis or recalcitrant conditions. If an infection develops, the use of occlusive dressings should be discontinued and appropriate antimicrobial therapy instituted.
HOW SUPPLIED:
| Analpram HC ® Cream 2.5% | 1 oz tube | (NDC 54766-799-04) |
| 12 x 4 gram tubes | (NDC 54766-799-65) | |
| 30 x 4 gram tubes | (NDC 54766-799-64) | |
| 1 oz Analpram Advanced Kit | (NDC 54766-732-04) | |
| 30 x 4 gram Analpram Advanced Kit | (NDC 54766-731-64) |
Rx Only
Manufactured for Sebela Ireland Ltd.
By Ferndale Laboratories, Inc., Ferndale, MI 48220 U.S.A.
Distributed by Sebela Pharmaceuticals Inc.
645 Hembree Parkway, Suite I
Roswell, GA 30076
www.sebelapharma.com
Toll Free 1-844-732-3521
PI 799640115
Rev. Jan. 2015
Aquaphor
® is a registered trademark of Beiersdorf AG.
Analpram HC
® is a registered trademark of Sebela International Limited.
#6994I
©2015 Reproduction prohibited
Product Information
VASCULERA ® Tablets for oral administration.
Dispensed by prescription.
diosmiplex 630 mg
VASCULERA is a specially formulated prescription medical food product for the clinical dietary management of the metabolic processes of chronic venous insufficiency. VASCULERA must be administered under physician supervision.
DESCRIPTION
VASCULERA (diosmiplex) consists of a specially formulated proprietary blend of micronized, highly purified diosmin glycoside in combination with alkaline granules, alka4-complex. Diosmin glycoside manages venous inflammation, accumulation of polymorphonuclear leukocytes, platelets and other thrombotic components as well as edema, caused by a deterioration of venous vessel walls. Alka4-complex works by buffering stomach acid and managing blood pH to affect local metabolic acidosis in veins.
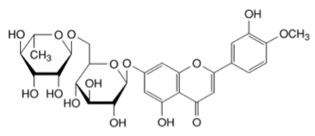
Diosmin glycoside
Each VASCULERA tablet contains 600 mg of diosmin glycoside (diosmin), a micronized, highly purified flavonoid fraction (from citrus) with hesperidoside constituents. In clinical trials, this level of intake has been shown to manage chronic venous insufficiency (CVI). Diosmin is chemically described as (7-[[6-O-(6-Deoxy-α-L-mannopyranosyl)-β-D-glucopyranosyl]oxy]-5-hydroxy-2-(3-hydroxy-4-methoxyphenyl)-4H-1-benzopyran-4-one) with a molecular weight of 608.5. The empirical formula for diosmin is C28H32O15. The structural formula is:
Alka4-complex
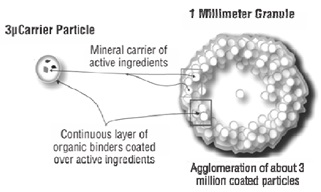
Each VASCULERA tablet contains 30 mg of a proprietary, structured, highly alkaline agglomeration of reactive magnesium hydroxide (Mg(OH)2, molecular weight 58.3), potassium chloride (KCl, molecular weight 74.6) and potassium hydroxide (KOH, molecular weight 56.1) held apart from each other in a matrix of calcium carbonate (CaCO3, molecular weight 100.1), microcrystalline cellulose and croscarmellose sodium. The agglomerated particle matrix has the following approximate characteristics:
Other Ingredients
VASCULERA contains the following other ingredients as fillers and excipients: microcrystalline cellulose, steric acid, croscarmellose sodium, povidone, silicon dioxide, hydroxypropyl methylcellulose, glycerine USP, tablet coating {Opadry ® II (contains maltodextrin)} and water. Tablets do not contain fructose, glucose, sucrose, lactose, gluten, tree nuts, peanuts, or flavors. VASCULERA is suitable for vegans.
Medical Foods
The U.S. Congress defined “medical food” in the Orphan Drug Act and Amendments of 1988 as “a food which is formulated to be consumed or administered enterally under the supervision of a physician, and which is intended for the specific dietary management of a disease or condition for which distinctive nutritional requirements, based on recognized scientific principles, are established by medical evaluation.” VASCULERA has been developed, manufactured and labeled in accordance with the statutory definition of a medical food. VASCULERA is to be used only under a physician’s supervision.
Generally Recognized As Safe
The ingredients in VASCULERA are Generally Recognized as Safe (GRAS). This is the statutory U.S. safety standard that the U.S. Food and Drug Administration (FDA) requires of all ingredients added to food products. The standard for an ingredient to achieve GRAS status requires technical demonstration of non-toxicity and safety, general recognition of safety through widespread usage and agreement of that safety by experts in the field.
CHRONIC VENOUS INSUFFICIENCY (CVI)
Chronic venous insufficiency is caused by venous hypertension (VH). VH, in turn, aggravates these metabolic imbalances leading to a self-perpetuating cycle of further metabolic changes, including venous acidosis. These changes promote further inflammation in vascular tissue leading to edema, skin damage and possible ulceration and deep vein thrombosis (DVT). Varicose veins and hemorrhoids are also part of the spectrum of CVI disorders.
CLINICAL PHARMACOLOGY
Mechanism of Action
VASCULERA acts by restoring toward normal the metabolic processes underlying the pathophysiology of CVI including modulation of venous tone and capillary resistance, management of lymphatic drainage and inflammation in the microcirculation. Preclinical and clinical data suggest that the diosmin glycoside in VASCULERA manages venous tone by increasing smooth muscle contractibility. In addition, diosmin has been shown to decrease edema by increasing lymphatic contractility and drainage. Finally, diosmin manages the oxidative and inflammatory factors induced by VH. Alka4-complex has been shown in preclinical and clinical studies to act as an acid buffering agent in the gut and to counteract the effects of increased acid production systemically.
Diosmin glycoside
The diosmin in VASCULERA has been shown in both cell and animal models to enhance venous tone by prolonging the post-synaptic response to norepinephrine (NE) and increasing the sensitivity of vascular smooth muscle to NE and calcium, even under conditions of acidosis. Diosmin inhibits the NE degrading enzyme catechol-O-methyltransferase (COMT). Diosmin has also been shown to manage lymphatic drainage and decrease edema by increasing lymphatic vessel contraction frequency and amplitude. Venous hypertension induces production of inflammatory factors such as prostaglandins and leukotrienes, metalloproteinases, cytokines, adhesion molecules and VEGF. Localization of inflammatory cells generates high levels of tissue destructive reactive oxygen species (ROS). Diosmin acts as a scavenger of ROS, inhibits 5-lipoxygenase and the production of prostaglandins E2 and thromboxane B2, which may help to protect endothelial cells from damage associated with inflammation and acute oxidative stress.
Alka4-complex
Clinical studies have shown that alka4-complex in VASCULERA can reduce acidosis generated under extreme exercise loads. The specially agglomerated matrix of alka4-complex has been shown to resist neutralization by acid solutions. This ability to survive the acid environment of the stomach and to be taken up in the intestine may account for its effect on blood pH during exercise. Alka4-complex may counter the local acidosis produced in CVI and work synergistically with diosmin to manage venous inflammation. In addition, the alka4-complex has been shown to reduce the acid producing effects of certain foods by buffering the acidity in the gastrointestinal tract, while allowing a sufficient portion of the complex to survive in the stomach, and is absorbed in the small intestine thereby being available to manage mild acidosis.
METABOLISM
Diosmin glycoside
Diosmin is hydrolyzed to its aglycone form, diosmetin, by intestinal microorganisms and enzymes in the intestinal lumen prior to absorption. Once it reaches the liver, diosmetin is extensively glucuronidated before entering the systemic circulation. Diosmetin may also be reduced to its corresponding flavanone aglycone, hesperetin, by intestinal bacteria in second pass metabolism. No diosmin is detected in the serum in animals or humans. Only the active form diosmetin, is detected after β-glucoronidase digestion. The phenolic acid products of diosmetin degradation are excreted in the urine, while unabsorbed/unmetabolized diosmin and diosmetin are excreted in the feces.
Alka4-complex
In contrast to sodium bicarbonate, alka4-complex has been shown in acid dissolution studies to release alkalizing salts in two gradual steps. Starting at a pH of 2, alka4-complex takes approximately 15 min to raise the solution pH to 6 whereas this occurs in seconds with bicarbonate. A further increase to a pH of 7 occurs over the next 60-90 min period. This biphasic, slow release characteristic gives alka4-complex seven times more pH neutralizing capacity compared to the same concentration of sodium bicarbonate. The molecular protection afforded by the proprietary matrix allows the alka4-complex to enter the intestinal tract where it is absorbed. Compared to bicarbonate ions, hydroxyl ions present in alka4-complex have been shown to be better absorbed by the intestinal lumen.
CLINICAL EXPERIENCE
Hepatic and Renal Effects
In multiple clinical studies, the effects of diosmin in VASCULERA on blood chemistry as well as hepatic and renal function were compared to placebo in healthy subjects and patients with CVI. No changes were noted in most studies with the exception of statistically significant decreases in creatinine levels in subjects taking diosmin in several clinical trials.
Cardiovascular Safety
No cardio-vascular adverse effects of the diosmin in VASCULERA have been observed in clinical trials. Small decreases in both systolic and diastolic blood pressure have been noted in several studies for CVI. When used as a pretreatment for coronary artery bypass grafting, troponin I and lactate dehydrogenase levels were significantly lower in the post-cardiopulmonary bypass period.
TOXICITY
Diosmin glycoside
In chronic and acute toxicity studies in mice, rats and primates in single oral doses up to 3,000 mg/kg body weight/day or as repeated oral doses up to 583 mg/kg body weight/day (representing approximately 180 and 35 times respectively, the recommended daily human dose) for up to 26 weeks, diosmin showed no effect on body weight, routine blood hematology or chemistry parameters. In animal studies, macroscopic and histologic examination of all major organ systems revealed no pathological changes. Two teratogenicity studies using 50 and 100 mg/kg/day of diosmin against placebo show no differences on fetal formation, number per litter, weight gain, or developmental milestones. Finally, acute dosing of up to 5000 mg/day in humans for 4 days produced no toxic effects.
Effects on Reproductive Tissues
The diosmin in VASCULERA showed no impairment of reproductive function or effects on reproductive tissue in the rat after administration of an oral dose representing 37 times the recommended daily human dose (1 tablet/day). Adverse effects regarding fertility, embryotoxicity and perinatal and postnatal development of the generation born from treated animals were not observed. Diosmin in VASCULERA was tested in human clinical trials for hemorrhoids during the third trimester of pregnancy and 4 weeks after birth. Administration of diosmin at 900 mg per day did not affect pregnancy, fetal development, birth weight or infant growth and feeding.
Alka4-complex
Single doses of alka4-complex granules (0.5, 2 and 5 mg/kg) were administered by oral gavage on day 1 to male and female 6-7 week old Sprague-Dawley rats. Body weight, behavior and food intake were assessed daily showing no effect over a 15 day period. On day 15, animals were sacrificed and gross organ pathology assessed. The No Observable Adverse Effect Level (NOAEL) of alka4-complex granules administered in a single oral dose was 5 mg/kg, based on the parameters evaluated, and the maximum tolerated single oral dose is greater than 5 mg/kg as shown in this animal study.
SPECIAL POPULATIONS
Pregnant or Nursing Women
VASCULERA (diosmiplex) has not been fully tested in pregnant or nursing women. Animal studies suggest that diosmin does not produce developmental abnormalities when consumed during pregnancy. A small number of pregnant women have consumed diosmin (up to 900 mg/day) in the third trimester and the first 4 weeks post-partum with no effect on the fetus or baby. Effects in infants of nursing mothers are uncertain for chronic dosing. Therefore, the decision to use VASCULERA (diosmiplex) is solely up to the discretion of the prescriber. Women capable of becoming pregnant should consult with their physician before taking VASCULERA.
INDICATIONS AND USAGE
Indications
VASCULERA is indicated for the clinical dietary management of the metabolic processes of chronic venous insufficiency (CVI). Conditions which occur due to CVI include varicose veins, spider veins, edema, deep vein thrombosis, stasis dermatitis, venous ulcers and hemorrhoids. Symptoms of CVI include heavy leg syndrome (dull aching discomfort, heaviness, cramping, itching and tingling in legs), pain that gets worse when standing, pain that gets better when legs are raised, swelling of the legs (edema), redness of the legs and ankles, skin color changes around the ankles and thickening of the skin on the legs and ankles (stasis dermatitis). VASCULERA must be used under the supervision of a physician.
Usage
VASCULERA should be taken as directed by a physician. See Dosage and Administration for additional information.
PRECAUTIONS AND CONTRAINDICATIONS
Hypersensitivity
VASCULERA is contraindicated for anyone having a hypersensitivity to any ingredient in the product. See “ Description and Other Ingredients” for a full list of ingredients.
Patients with Cancer
Diosmin (up to 900 mg/day) has been administered to a small number of breast cancer patients who were experiencing lymphedema following surgical and nodal irradiation treatment with resultant reduction of arm edema but no effect on the cancer. Animal studies and more than 20 years of clinical use in Europe have not found any evidence of carcinogenicity or mutagenicity when the components of Vasculera are used as recommended. Since no specific studies with VASCULERA (diosmiplex) have been performed in this population, the decision to use Vasculera in this patient population is solely up to the discretion of the prescriber.
ADVERSE EVENTS
Diosmin glycoside
Many clinical trials have been conducted in adults with various manifestations of CVI to assess the efficacy of orally administered diosmin in doses ranging from 400 to 5,000 mg/day for up to a year. No serious adverse events were reported in any of the studies. Commonly reported adverse events included gastrointestinal disturbances and headaches; these were generally mild in severity and did not usually result in patients discontinuing participation in the study. The following adverse events (and approximate percentages) were reported in clinical trials but their frequency did not differ from placebo: rash (1%), cramping in lower limb (2%), phlebitis (2%), venous thrombosis (4%), and skin changes around existing ulcer, swelling of the extremities and body rash (1.6%). Dyspepsia, or non-specific mild stomach upset, occurred in up to 7% of subjects taking diosmin at various doses and was seen with approximately twice the frequency seen in the placebo groups. Rare adverse events include inguinal pain, cystitis, asthenia, metrorrhagia and menometrorrhagia. In clinical trials, the incidence of adverse events in elderly populations (≥70 years of age) was not significantly different from that in younger populations nor were adverse events higher in patients with concomitant hypertension, atherosclerosis, diabetes, neurologic/psychiatric disease or alcoholism.
PHARMACOKINETICS
Diosmin glycoside
The pharmacokinetic parameters of diosmin have been studied in humans as well as several animal species (i.e., rats, dogs, rabbits, and monkeys). The pharmacokinetics of diosmin administered to 5 healthy subjects (2 men and 3 women), 20 to 45 years of age, at a single oral dose of ~600 mg was determined. Blood samples were taken at 0.5, 1, 2, 4, 8, 12, 24, and 48 hours after administration. Urine was collected at baseline, between 0 and 24 hours, and 24 and 48 hours after administration. All samples were subjected to incubation with β-glucuronidase prior to assessment. No diosmin was detected in the plasma (detection limit of 20 ng/mL). Only diosmetin, the aglycone of diosmin, was found in the plasma, with a peak concentration (Tmax) of 400 ng/mL after 1 hour. Plasma levels decreased slowly after 2 hours, constantly after 24 hours, and were still detectable after 48 hours. The serum half-life (t1/2) among all subjects was 31.5 ± 8.6 hours. The pharmacokinetic parameters of this study are shown below:
| Parameters | Mean ± SD | Parameters | Mean ± SD | |
| Cmax (ng/mL) | 417 ± 94.1 | AUC(0-48h) ng/mL.h | 5617.1 ± 1518.4 | |
| T1/2 (h) | 31.5 ± 8.6 | TCL (L/h)* | 1.32 ± 0.42 | |
| MRT (h) | 36.6 ± 9.9 | Vd (L)1 | 62.1 ± 7.9 |
Abbreviations: AUC, area under the curve; Cmax, maximum concentration; MRT, mean residence time; T1/2, half-life; TCL, total clearance; Vd, volume of distribution.
1Total clearance and volume of distribution were computed assuming complete bioavailability.
CLINICAL STUDIES
Dietary Management of Chronic Venous Insufficiency
In addition to more than 2 decades of clinical use in multiple countries, more than 40 clinical trials comprising >15,000 patients have been published. In studies using clinical endpoints of patient reported symptoms, edema and quality of life, diosmin consistently demonstrated 30- 60% superiority to placebo or standard of care. The largest of the published CVI studies, a 2- year trial, enrolled 5,052 subjects in 23 countries, all of whom had CVI symptoms. Of those enrolled, 43% had reflux demonstrable with Doppler examination. All subjects were in CEAP (Clinical signs, Etiology, Anatomic distribution, and Pathological dysfunction) class 0-4 corresponding to mild-moderate disease without ulceration. The female/male ratio was 4/1, mean age 45 years and most were physically active. Leg heaviness was the most frequent complaint at baseline followed by aching, swelling and nocturnal cramping. CEAP class correlated with age. After 6 months, there was a significant reduction in CEAP class, edema and quality of life (QOL) score (p=0.0001 for all) irrespective of whether or not reflux was demonstrable at baseline. At the end of 6 months, 79% of subjects and 83% of investigators rated the effectiveness of diosmin as good or excellent irrespective of whether or not subjects regularly wore compression stockings. Studies using objective endpoints such as venous reflux, venous and lymphatic flow measured by techniques such as scintographic Doppler, plethysmography and venous pressure have shown similar statistically significant improvement in all parameters, generally in the range of 20-40% better than placebo. Other studies have confirmed the advantage of the micronized form of diosmin over the unmicronized form with regard to reduction in edema and clinical symptoms.
Dietary Management of Hemorrhoids
Multiple studies involving several hundred patients have been reported. These have routinely shown that diosmin reduces symptoms of discomfort, swelling, inflammation and time to resolution when compared to standard of care. In general, resolution time of acute flares is shortened from 8 days to about 4-5 days. A double-blind, placebo-controlled study (n=120) showed statistically better management of pain, pruritus, discharge, edema, erythema, and bleeding on examination. One study (n=351) compared the efficacy of combining infrared photocoagulation (IRP) and diosmin vs each therapy used alone on bleeding cessation in patients with grades I, II, and III acute internal hemorrhoids. The percentage of patients with no bleeding after 5 days of therapy was higher in the combined group (75%) compared with only diosmin (60%) or with IRP alone (56%). Patients with grades I and II hemorrhoids responded significantly better (83% and 62%, respectively) to either therapy than those with grade III hemorrhoids (23%). Patients experiencing an acute hemorrhoidal episodes for less than 48 h were enrolled in a randomized, double-blind, placebo-controlled study. One group received diosmin (n=49) and the other a placebo (n=41). During the 7-day administration, there was a significant difference in favor of diosmin in the dietary management of discomfort, edema and bleeding. A study has compared the results of the conservative management of hemorrhoids between diosmin and sclerotherapy (SCL), in terms of the subjective and objective outcome of patients after a follow-up period of 2 years. Average Symptoms Score (ASS) and Average Anoscopy Scores (AAS) decreased in both of the groups in the first three visits. At the end of the second visit, ASS and AAS significantly declined in the diosmin group. In addition, ASS and AAS decreased to the nadir level in both groups at the end of the 26th week. In another study of 100 patients with endoscopically confirmed hemorrhoids, patients receiving diosmin showed an improvement in discomfort, anal discharge, proctitis and time to resolution compared with placebo (p< 0.001).
Dietary Management of Venous Ulcers
More than 20 clinical trials involving >1000 patients have been published. In general, these compare treatment using diosmin with standard of care (compression and dressing) and tend to be open label. Most show improvement within 2-4 months. Diosmin uniformly increased the number of resolved ulcers and the time to resolution for ulcers <10 cm but had little benefit for ulcers >10 cm. Diosmin also deceased the length, but not the number, of hospital stays. Several randomized trials (n=107-150) compared ulcer resolution and time on diosmin plus standard of care (SOC) vs SOC alone or with placebo. In each case, the patients administered diosmin had a better outcome than the comparators. Ulcer resolution varied with the size of the ulcer with about 70% resolution of ulcers <3 cm versus 50% in the SOC group while ulcers between 3-6 cm showed resolution rates of 60% and 30%, respectively. A meta-analysis of seven comparable prospective studies (n=723) found a significant difference in overall ulcer resolution between diosmin administered groups and controls at 2 months (p=0.0088). By 6 months, the difference, while still significant, had narrowed to 61% vs. 48% for placebo (OR=2.02, p=0.035), the difference representing the fact that persistent ulcers tend to be larger and deeper and have more vascular compromise. A meta-analysis of 5 European trials (n=723) found that for ulcers between 5-10 cm, diosmin, together with SOC, significantly reduced the time to clearance, the percent of ulcers resolved and was especially valuable for ulcers that had been present for 6-12 months.
Concomitant Use
No evidence of drug incompatibility, drug interaction, or photosensitizing action of diosmin was observed when combined with drugs used to treat a wide variety of clinical disorders. Although grapefruit juice is known to affect metabolism of many drugs by the CYP450 enzyme system, this effect has not been ascribed specifically to diosmin or hesperidin. Hesperidin, the flavonoid from which diosmin is derived, is thought to increase bioavailability of diltiazem by a combination of CYP450 3A4 inhibition and increased enteric absorption. In single dose PK studies, diosmin significantly delayed the T1/2 and increased the AUC of chlorzoxazone, possibly by interfering with CYP2E1 metabolism. Similar effects have been noted with diclofenac and metronidazole, both of which are metabolized by the CYP2C9 enzyme system.
OVER USAGE
Diosmin glycoside
There are no known cases of over usage of the diosmiplex in VASCULERA. Animal studies have shown that consuming the equivalent of 56 VASCULERA tablets containing 600 mg of diosmin or 30 mg of alka4-complex at one time did not produce adverse events. However, as in most over usage situations, symptoms following an over usage of VASCULERA could vary according to the patient. If an over usage occurs, patients should be managed by systematic and supportive care as soon as possible following product consumption.
PRODUCT ADMINISTRATION
The recommended intake of VASCULERA (diosmiplex) is 1 tablet per day for the dietary management of Chronic Venous Insufficiency (CVI), manifested as: varicose/spider veins, edema, stasis dermatitis and or venous ulcers. Results may not be seen for 4 to 8 weeks. For venous ulcers, results may not be seen for several months. For symptomatic flares of CVI manifested as hemorrhoidal disease, the recommended initial intake is 1 tablet 3 times daily for 4 days followed by 1 tablet twice daily for 9 days, or as directed by a physician. The chronic management of hemorrhoidal disease requires a maintenance intake of 1 tablet of Vasculera daily.
HOW SUPPLIED
VASCULERA is a uniformly coated oblong tablet, beige in color, with a V debossed at one end of the tablet and a 6 debossed at the other end of the tablet.
The tablets are packaged in unit-of-use blister packages:
68040-610-14 contents 30 tablets per carton (30-day supply)
68040-610-10 contents 15 tablets per carton (15-day supply sample)
68040-610-03 contents 6 tablets per carton (6-day supply sample)
Distributed by: Primus Pharmaceuticals, Inc.
Scottsdale, AZ 85253 (480) 483-1410, www.primusrx.com
Manufactured by: Cornerstone Research and Development
Ogden, UT 84404
www.vasculera.com
U.S. Patent Nos. 6,066,342; 6,143,221, and 6,270,708 & patents pending under license from pH Science Holdings, Inc.
© 2013 Primus Pharmaceuticals, Inc. All rights reserved. ISS. 1213 # 16005
AloeClean ®
1% ALOE POLYSACCHARIDES(AMAP 80/1300 kDa)
1% PELA POLYCOSANOL
advanced gentle cleansing wipe with aloe
PRIMARY INGREDIENTS: Each wipe contains 1% aloe polysaccharides (AMAP 80/1300 kDa) and 1% pela polycosanol.
OTHER INGREDIENTS: ceteareth 20, cetyl alcohol, methyl paraben, propyl paraben, purified water, sodium citrate, sodium lauryl sulfate, and stearyl alcohol.
| ANALPRAM ADVANCED
hydrocortisone acetate and pramoxine hydrochloride kit |
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
| ANALPRAM ADVANCED
hydrocortisone acetate and pramoxine hydrochloride kit |
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
| ANALPRAM ADVANCED
hydrocortisone acetate and pramoxine hydrochloride kit |
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
| Labeler - Sebela Pharmaceuticals Inc. (079104574) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Ferndale Laboratories, Inc. | 005320536 | analysis(54766-799, 54766-730, 54766-731, 54766-732) , manufacture(54766-732, 54766-799, 54766-730, 54766-731) , label(54766-799, 54766-730, 54766-731, 54766-732) | |