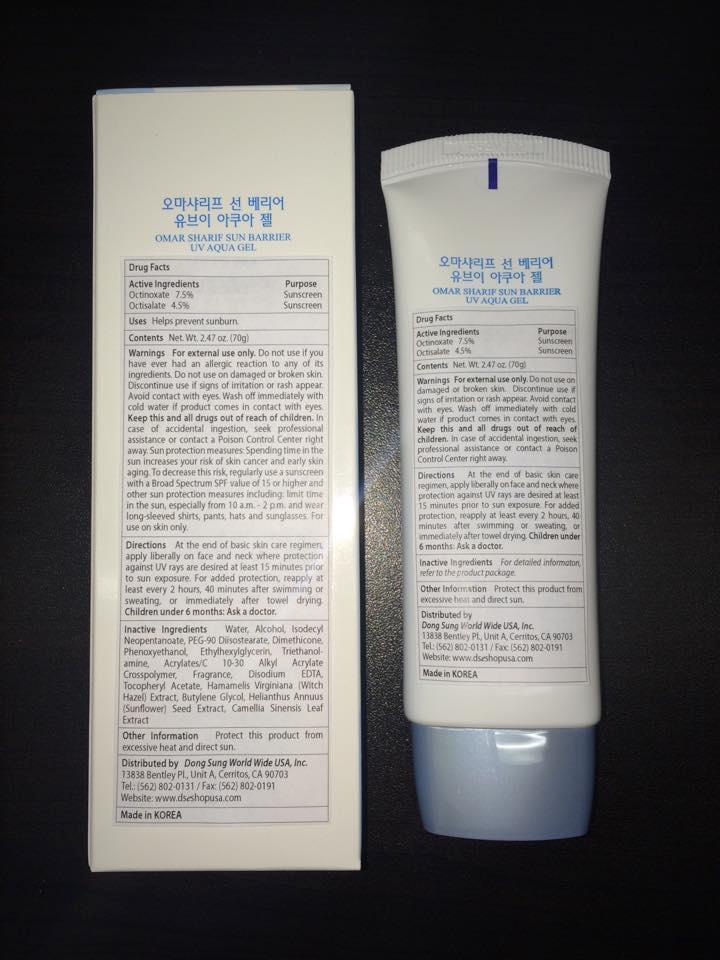
SUN BARRIER UV AQUA GEL BROAD SPECTRUM SPF 12- octinoxate and octisalate lotion
Dong Sung Pharm. Co., Ltd.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Warnings For external use only. Do not use if you have ever had an allergic reaction to any of its ingredients. Do not use on damaged or broken skin. Discontinue use if signs of irritation or rash appear. Avoid contact with eyes. Wash off immediately with cold water if product comes in contact with eyes. Keep this and all drugs out of reach of children. In case of accidental ingestion, seek professional assistance or contact a Poison Control Center right away. Sun protection measures: Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including: limit time in the sun, especially from 10 a.m. - 2 p.m. and wear long-sleeved shirts, pants, hats and sunglasses. For use on skin only.
At the end of basic skin care regimen, apply liberally on face and neck where protection against UV rays are desired at least 15 minutes prior to sun exposure. For added protection, reapply at least every 2 hours, 40 minutes after swimming or sweating, or immediately after towel drying. Children under 6 months: Ask a doctor.
Water, Alcohol, Isodecyl Neopentanoate, PEG-90 Diisostearate, Dimethicone, Phenoxyethanol, Ethylhexylglycerin, Triethanolamine, Acrylates/C 10-30 Alkyl Acrylate Crosspolymer, Fragrance, Disodium EDTA, Tocopheryl Acetate, Hamamelis Virginiana (Witch Hazel) Extract, Butylene Glycol, Helianthus Annuus (Sunflower) Seed Extract, Camellia Sinensis Leaf Extract
Protect this product from excessive heat and direct sun.
Dong Sung Pharm. Co., Ltd.