DIGICLEAN- triclosan solution
Ecolab Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
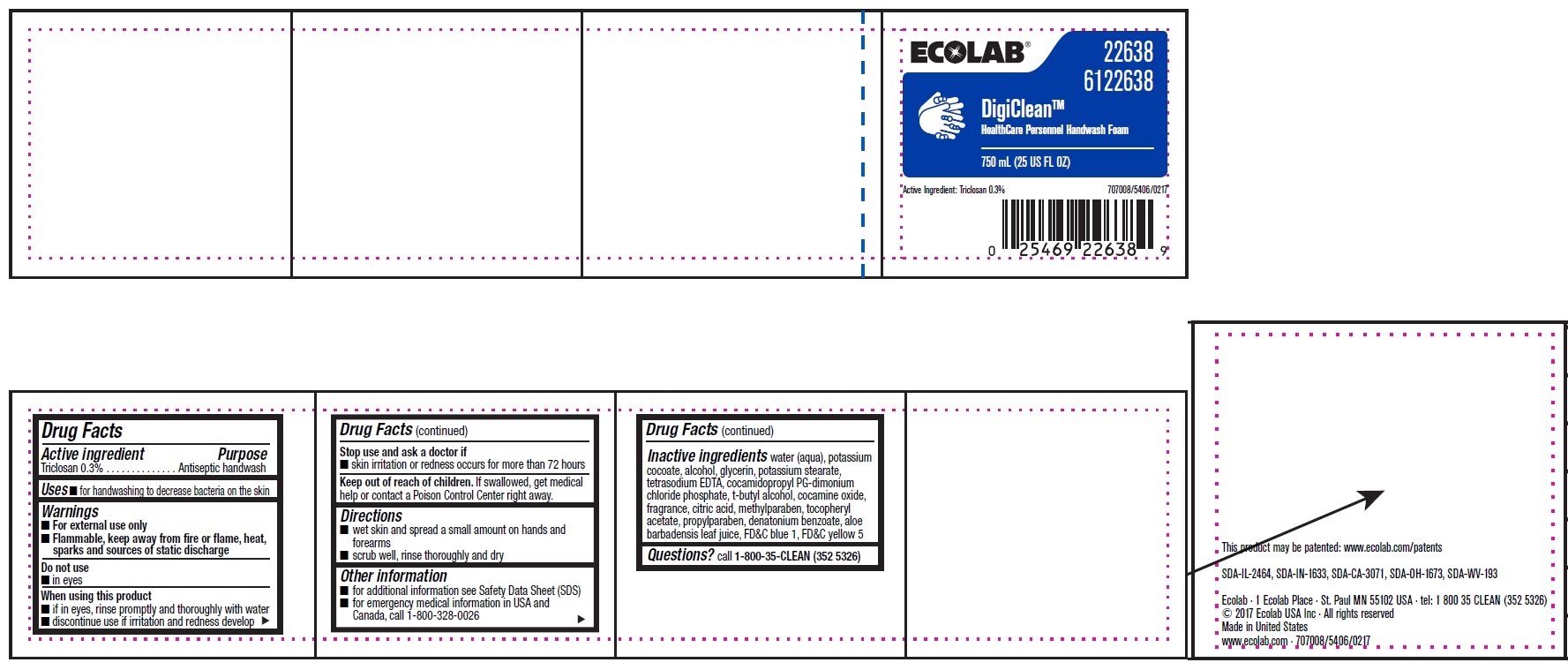
Drug Facts
Warnings
- For external use only
- Flammable, keep away from fire or flame, heat, sparks and sources of static discharge
Directions
- wet skin and spread a small amount on hands and forearms
- scrub well, rinse throughly and dry
Other information
- for additional information, see Safety Data Sheet (SDS)
- for emergency medical information in USA and Canada, call 1-800-328-0026
Inactive ingredients water (aqua), potassium cocoate, alcohol, glycerin, potassium stearate, tetrasodium EDTA, cocamidopropyl PG-dimonium chloride phosphate, t-butyl alcohol, cocamine oxide, fragrance, citric acid, methylparaben, tocopheryl acetate, propylparaben, denatonium benzoate, aloe barbadensis leaf juice, FD&C blue 1, FD&C yellow 5
Principal Display Panel and Representative Label
ECOLAB
DigiClean™
Healthcare Personnel Handwash Foam
750 mL/ (25 US FL OZ)
Active Ingredient: Triclosan 0.3% 707008/5406/0217
This product may be patented: www.ecolab.com/patents
SDA-IL-2464, SDA-IN-1633, SDA-CA-3071, SDA-OH-1673, SDA-WV-193
Ecolab · 1 Ecolab Place · St. Paul MN 55102 USA · tel: 1 800 35 CLEAN (352 5326)
© 2017 Ecolab USA Inc · All rights reserved
Made in United States
www.ecolab.com · 707008/5406/0217
| DIGICLEAN
triclosan solution |
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
| Labeler - Ecolab Inc. (006154611) |