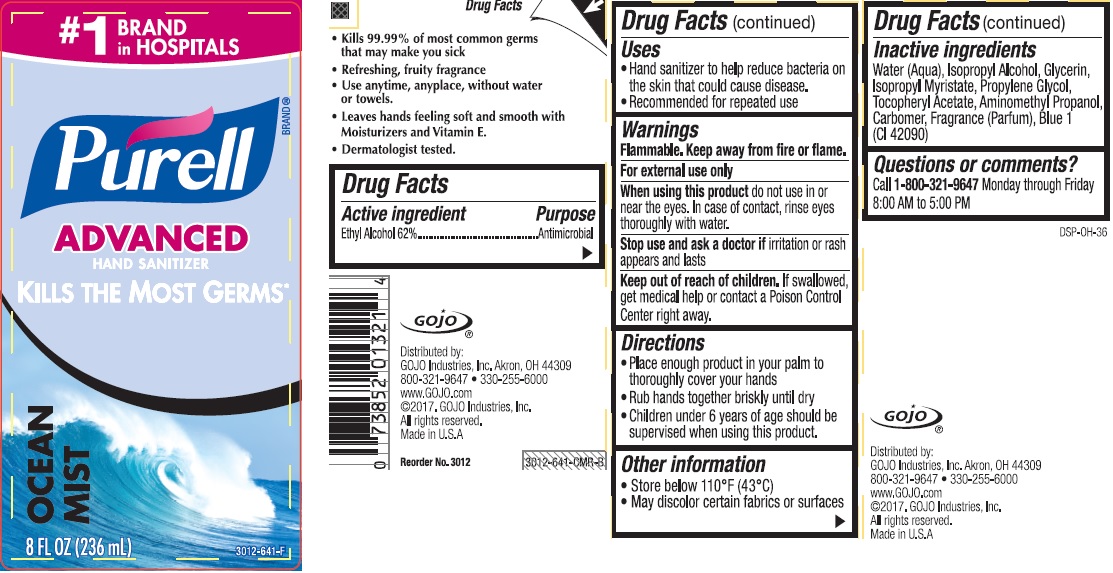
PURELL INSTANT HAND SANITIZER OCEAN MIST- alcohol gel
GOJO Industries, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
PURELL® Instant Hand Sanitizer Ocean Mist
Use
- Hand sanitizer to help reduce bacteria on the skin that could cause disease
- Recommended for repeasted use
Warnings
Flammable. Keep away from fire or flame.
For external use only
Directions
- Place enough product in your palm to thoroughly cover your hands
- Rub hands together briskly until dry
- Children under 6 years of age should be supervised when using this product.
| PURELL INSTANT HAND SANITIZER OCEAN MIST
alcohol gel |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Labeler - GOJO Industries, Inc. (004162038) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| GOJO Industries, Inc. | 036424534 | manufacture(21749-510) | |
Revised: 8/2020
Document Id: 283b05cc-21b5-4767-b753-85e5ac5cc522
Set id: 2c60726a-8480-41b7-ac90-4522210fad58
Version: 2
Effective Time: 20200831
GOJO Industries, Inc.