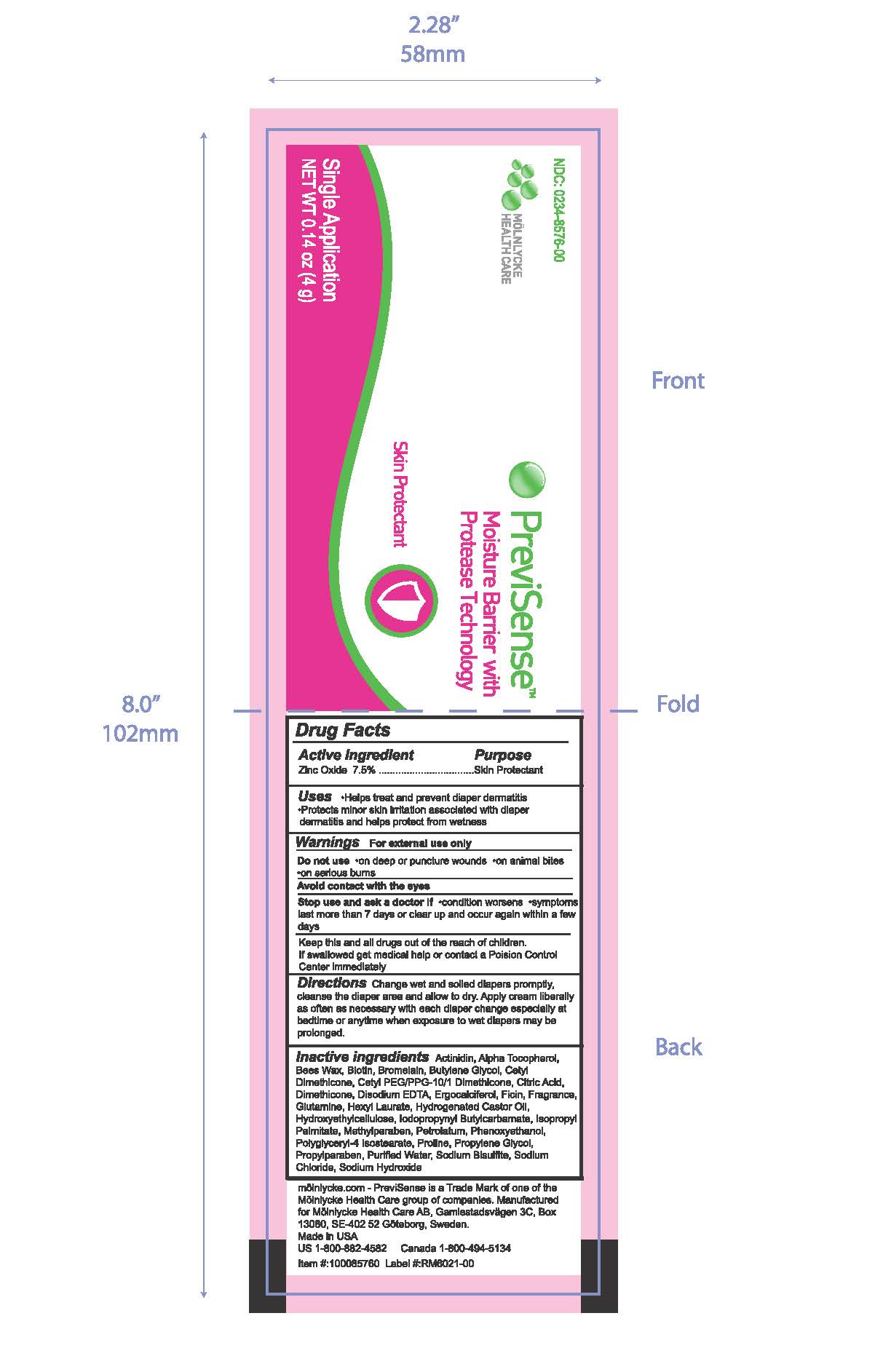
PREVISENSE MOISTURE BARRIER WITH PROTEASE TECHNOLOGY- zinc oxide 7.5% cream
Molnlycke Health Care
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
PreviSense Moisture Barrier with Protease Technology Sachet
Uses
- Helps treat and prevent diaper dermatitis
- Protects minor skin irritation associated with diaper dermatitis and helps protect from wetness
Stop use and ask a doctor if
- condition worsens
- symptoms last more than 7 days or clear up and occur again within a few days
Keep this and all drugs out of the reach of children. If swallowed, get medical help or contact a Poison Control Center immediately.
Directions
Change wet and soiled diapers promptly, cleanse the diaper area and allow to dry. Apply cream liberally as often as necessary with each diaper change especially at bedtime or anytime when exposure to wet diapers may be prolonged.
Inactive Ingredients
Actinidin, Alpha Tocopherol, Bees Wax, Biotin, Bromelain, Butylene Glycol, Cetyl Dimethicone, Cetyl PEG/PPG-10/1 Dimethicone, Citric Acid, Dimethicone, Disodium EDTA, Ergocalciferol, Ficin, Fragrance, Glutamine, Hexyl Laurate, Hydrogenated Castor Oil,
Hydroxyethylcellulose, Iodopropynyl Butylcarbamate, Isopropyl Palmitate, Methylparaben, Petrolatum, Phenoxyethanol, Polyglyceryl-4 Isostearate, Proline, Propylene Glycol, Propylparaben, Purified Water, Sodium Bisulfite, Sodium
Chloride, Sodium Hydroxide
| PREVISENSE MOISTURE BARRIER WITH PROTEASE TECHNOLOGY
zinc oxide 7.5% cream |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Molnlycke Health Care (165301032) |