LANEIGE SKIN VEIL NO.21 SPF 26 PAPLUS- octinoxate, titanium dioxide, and zinc oxide lotion
LANEIGE SKIN VEIL NO.23 SPF 26 PAPLUS- octinoxate, titanium dioxide, and zinc oxide lotion
LANEIGE SKIN VEIL NO.31 SPF 26 PAPLUS- octinoxate, titanium dioxide, and zinc oxide lotion
LANEIGE SKIN VEIL NO.33 SPF 26 PAPLUS- octinoxate, titanium dioxide, and zinc oxide lotion
AMOREPACIFIC CORPORATION
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
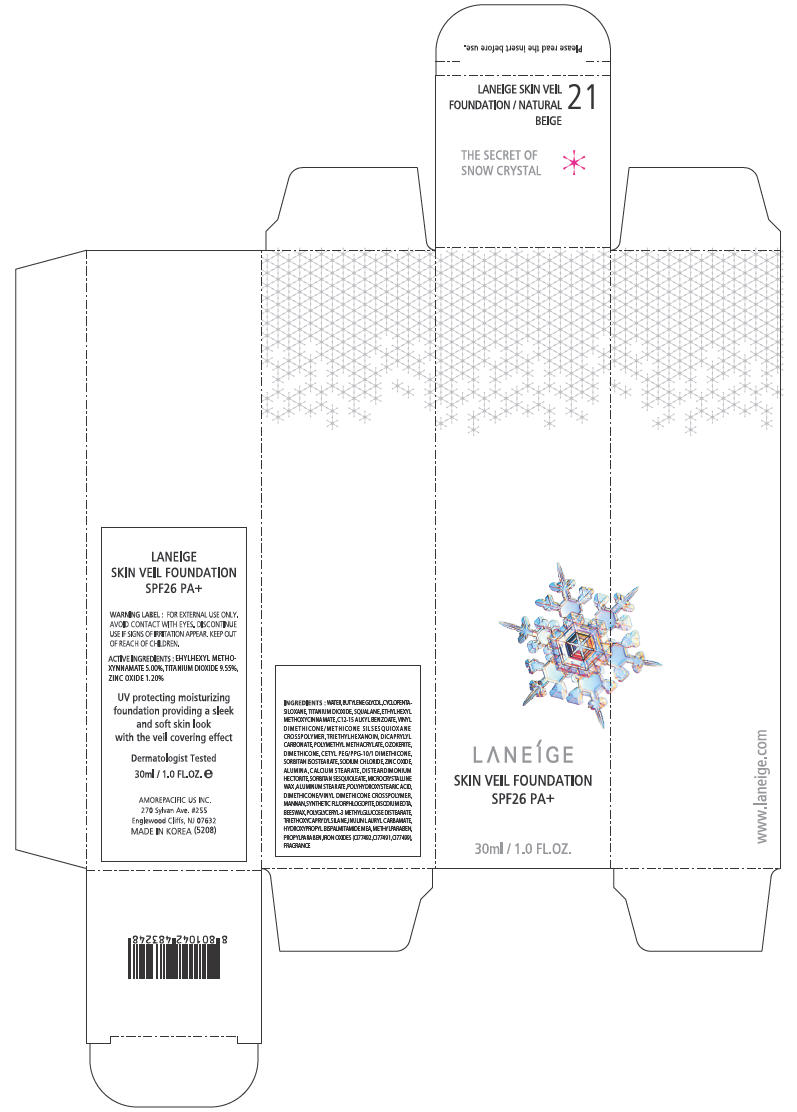
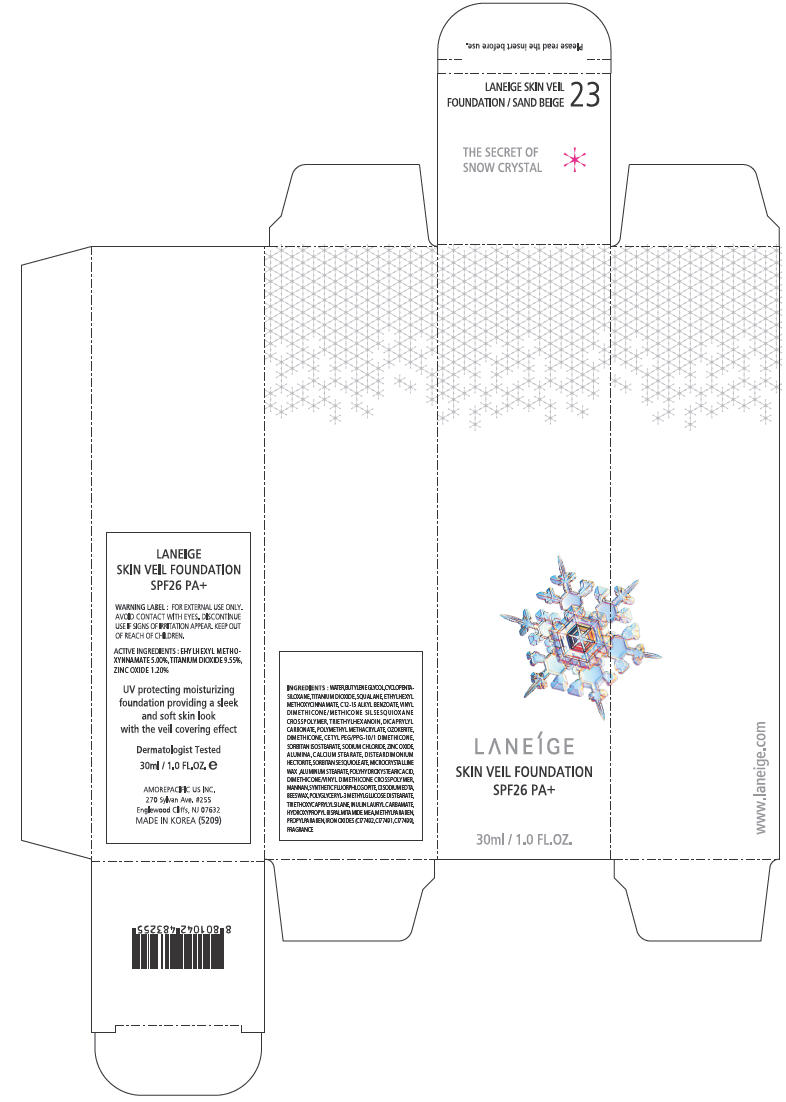
WARNING LABEL
FOR EXTERNAL USE ONLY. AVOID CONTACT WITH EYES.
DISCONTINUE USE IF SIGNS OF IRRITATION APPEAR.
KEEP OUT OF REACH OF CHILDREN.
ACTIVE INGREDIENTS
EHYLHEXYL METHOXYNNAMATE 5.00%, TITANIUM DIOXIDE 9.55%, ZINC OXIDE 1.20%
UV protecting moisturizing foundation providing a sleek and soft skin look with the veil covering effect
INGREDIENTS
WATER, BUTYLENE GLYCOL, CYCLOPENTASILOXANE, TITANIUM DIOXIDE, SQUALANE, ETHYLHEXYL METHOXYCINNAMATE, C12-15 ALKYL BENZOATE, VINYL DIMETHICONE/METHICONE SILSESQUIOXANE CROSSPOLYMER, TRIETHYLHEXANOIN, DICAPRYLYL CARBONATE, POLYMETHYL METHACRYLATE, OZOKERITE, DIMETHICONE, CETYL PEG/PPG-10/1 DIMETHICONE, SORBITAN ISOSTEARATE, SODIUM CHLORIDE, ZINC OXIDE, ALUMINA, CALCIUM STEARATE, DISTEARDIMONIUM HECTORITE, SORBITAN SESQUIOLEATE, MICROCRYSTALLINE WAX ,ALUMINUM STEARATE, POLYHYDROXYSTEARIC ACID, DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER, MANNAN, SYNTHETIC FLUORPHLOGOPITE, DISODIUM EDTA, BEESWAX, POLYGLYCERYL-3 METHYLGLUCOSE DISTEARATE, TRIETHOXYCAPRYLYLSILANE, INULIN LAURYL CARBAMATE, HYDROXYPROPYL BISPALMITAMIDE MEA, METHYLPARABEN, PROPYLPARABEN, IRON OXIDES (CI77492, CI77491, CI77499), FRAGRANCE
AMOREPACIFIC US INC.
270 Sylvan Ave. #255
Englewood Cliffs, NJ 07632
PRINCIPAL DISPLAY PANEL - 30ml Bottle Carton - NO. 21
LANEÍGE
SKIN VEIL FOUNDATION
SPF26 PA+
30ml / 1.0 FL.OZ.
PRINCIPAL DISPLAY PANEL - 30ml Bottle Carton - NO. 23
LANEÍGE
SKIN VEIL FOUNDATION
SPF26 PA+
30ml / 1.0 FL.OZ.
PRINCIPAL DISPLAY PANEL - 30ml Bottle Carton - NO. 31
LANEÍGE
SKIN VEIL FOUNDATION
SPF26 PA+
30ml / 1.0 FL.OZ.
PRINCIPAL DISPLAY PANEL - 30ml Bottle Carton - NO. 33
LANEÍGE
SKIN VEIL FOUNDATION
SPF26 PA+
30ml / 1.0 FL.OZ.