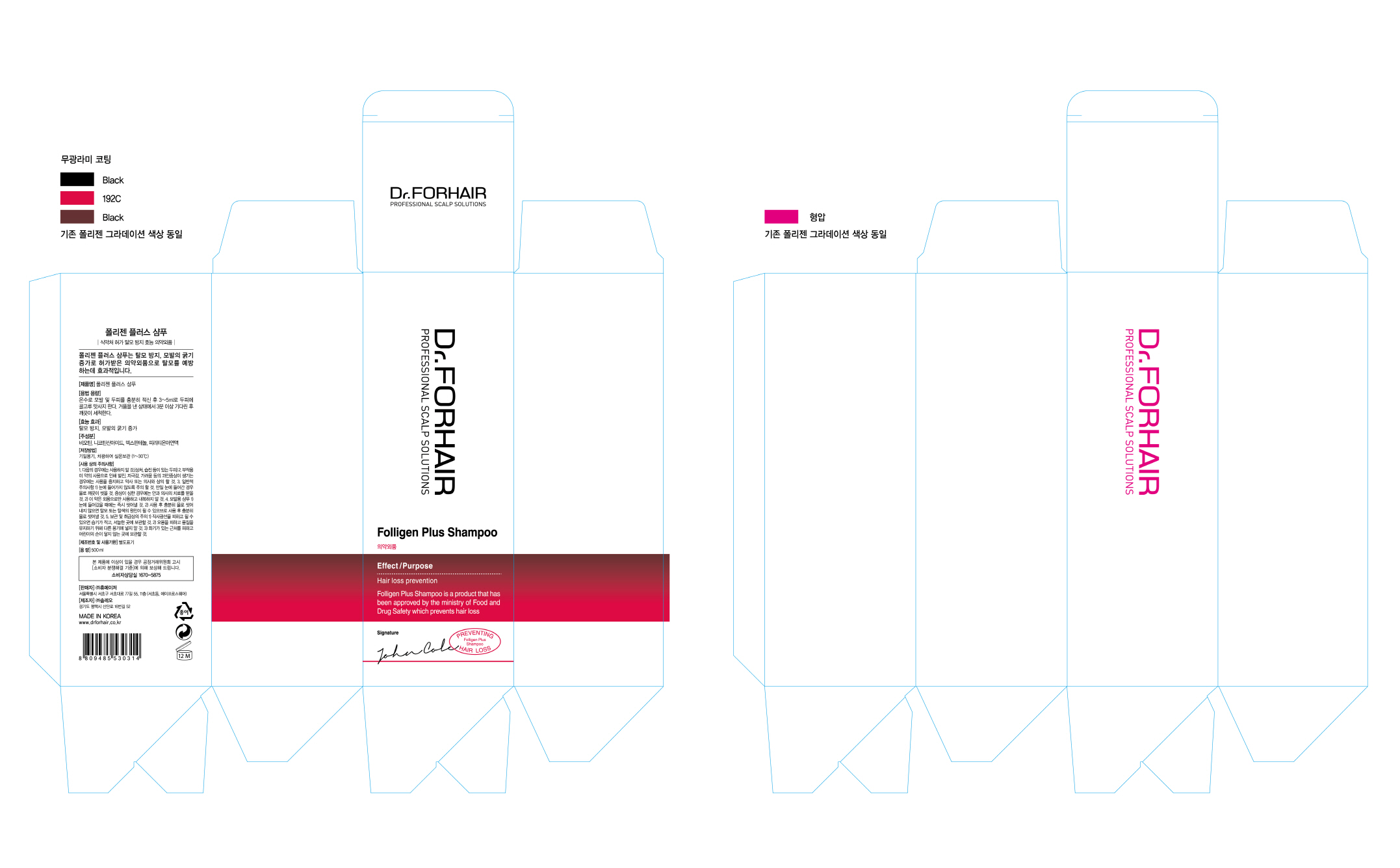
FOLLIGEN PLUS- pyrithione zinc shampoo
Humajor Co., Ltd.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Drug Facts
When using this product do not get into eyes. If contact occurs, rinse eyes thoroughly with water.
Stop use and ask a doctor. If condition worsens or does not improve after regular use as directed.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Keep it in airtight container in room temperature(1~30degree C)
| FOLLIGEN PLUS
pyrithione zinc shampoo |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - Humajor Co., Ltd. (689061827) |
| Registrant - Humajor Co., Ltd. (689061827) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Humajor Co., Ltd. | 689061827 | manufacture(69244-0002) | |
Revised: 5/2018
Document Id: 6b65880b-7e75-115c-e053-2a91aa0ab01b
Set id: 2b1b138f-67c0-423d-8aa8-630ff826ee21
Version: 5
Effective Time: 20180504
Humajor Co., Ltd.