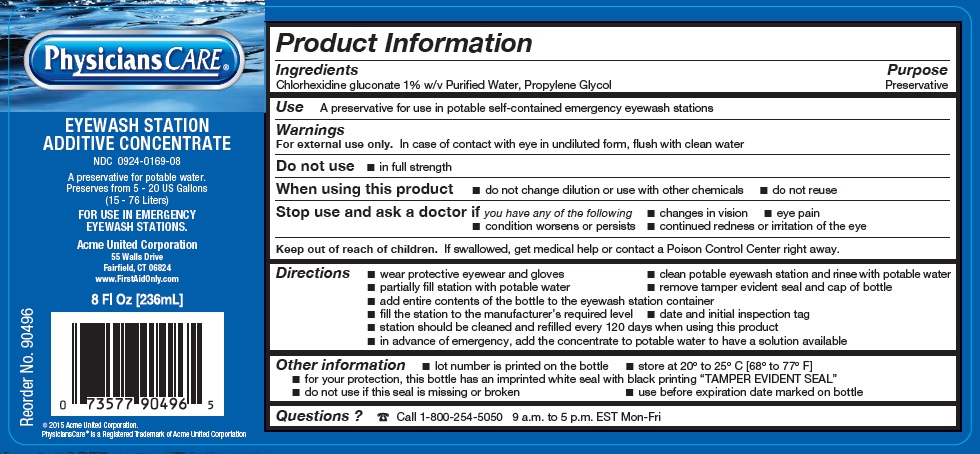
Label: PHYSICIANSCARE EYEWASH STATION ADDITIVE CONCENTRATE- chlorhexidine gluconate and propylene glycol liquid solution
-
Contains inactivated NDC Code(s)
NDC Code(s): 0924-0169-08 - Packager: Acme United Corporation
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated November 4, 2019
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
-
WARNINGS
Warnings
For external use only. In case of contact with eye in undiluted form, flush with clean water
Do not use
- in full strength
When using this product
- do not change dilution or use with other chemicals
- do not reuse
Stop use and ask a doctor ifyou have any of the following
- changes in vision
- eye pain
- condition worsens or persists
- continued redness or irritations of the eye
- KEEP OUT OF REACH OF CHILDREN
-
DOSAGE & ADMINISTRATION
Directions
- wear protective eyewear and gloves
- clean potable eyewash station and rinse with potable water
- partially fill station with potable water
- remove tamper evident seal and cap of bottle
- add entire contents of the bottle to the eyewash station container
- fill the station to the manufacturer's required level
- date and initial inspection tag
- station should be cleaned and refilled every 120 days when using this product
- in advance of emergency, add the concentrate to potable water to have solution available
- INFORMATION FOR PATIENTS
- QUESTIONS
- INACTIVE INGREDIENT
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
PHYSICIANSCARE EYEWASH STATION ADDITIVE CONCENTRATE
chlorhexidine gluconate and propylene glycol liquid solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0924-0169 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PROPYLENE GLYCOL (UNII: 6DC9Q167V3) (PROPYLENE GLYCOL - UNII:6DC9Q167V3) PROPYLENE GLYCOL 280 kg in 2800 L CHLORHEXIDINE GLUCONATE (UNII: MOR84MUD8E) (CHLORHEXIDINE - UNII:R4KO0DY52L) CHLORHEXIDINE GLUCONATE 145.6 kg in 2800 L Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) 2374.4 L in 2800 L Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0924-0169-08 0.236 L in 1 BOTTLE, UNIT-DOSE; Type 0: Not a Combination Product 01/26/2016 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part349 01/26/2016 Labeler - Acme United Corporation (001180207) Establishment Name Address ID/FEI Business Operations Acme United Corporation 045924339 relabel(0924-0169) , repack(0924-0169) Establishment Name Address ID/FEI Business Operations Acme United Corporation 080119599 repack(0924-0169) , relabel(0924-0169) Establishment Name Address ID/FEI Business Operations Niagara Pharmaceuticals, Inc. 205477792 manufacture(0924-0169)