BISCOLAX- bisacodyl suppository
Rugby Laboratories, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Biscolax
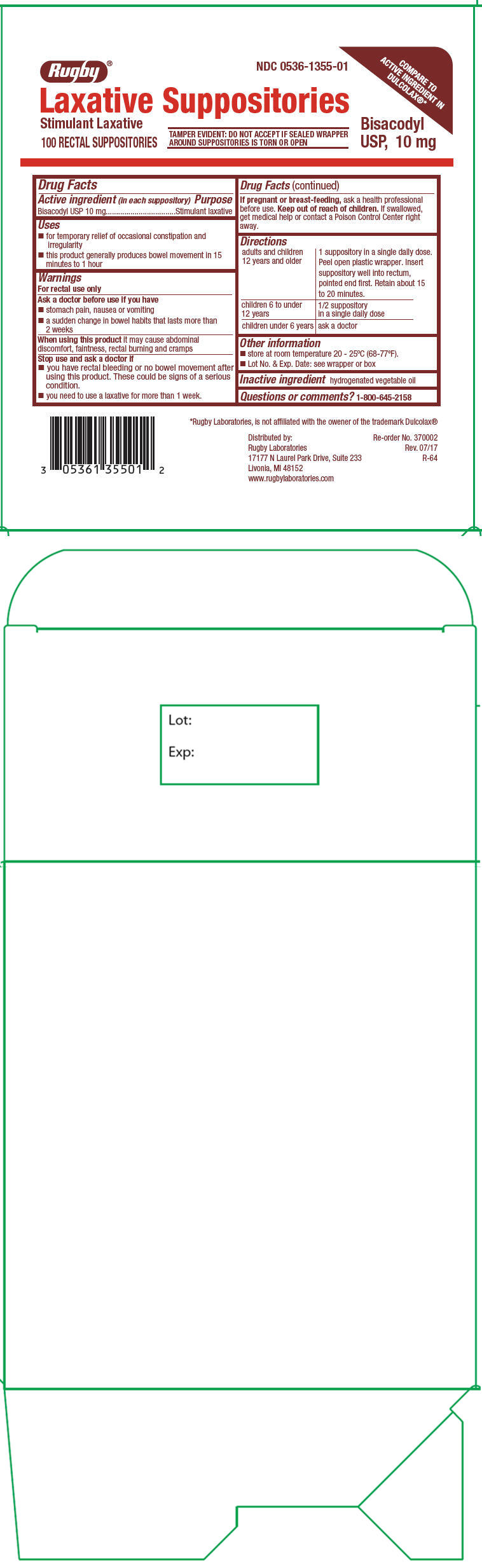
Uses
- for temporary relief of occasional constipation and irregularity
- this product generally produces bowel movement in 15 minutes to 1 hour
Warnings
For rectal use only
Ask a doctor before use if you have
- stomach pain, nausea or vomiting
- a sudden change in bowel habits that lasts more than 2 weeks
Directions
| adults and children 12 years and older | 1 suppository in a single daily dose. Peel open plastic wrapper. Insert suppository well into rectum, pointed end first. Retain about 15 to 20 minutes. |
| children 6 to under 12 years | 1/2 suppository in a single daily dose |
| children under 6 years | ask a doctor |
| BISCOLAX
bisacodyl suppository |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Rugby Laboratories, Inc. (079246066) |
Revised: 9/2019
Document Id: 936414cb-0ab7-c576-e053-2a95a90a51f9
Set id: 2a1b271e-ae17-4284-bfa1-4d0e3c75fcc3
Version: 4
Effective Time: 20190925
Rugby Laboratories, Inc.