Label: CVS HEALTH COLD AND HOT ROLL-ON- lidocaine, menthol cream
- NDC Code(s): 66902-156-25
- Packager: NATURAL ESSENTIALS, INC.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated June 1, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredients
- Purpose
- Uses
-
Warnings
For external use only
- Avoid contact with eyes or mucous membranes
- Do not apply to open wounds or damaged skin
- Do not bandage or use heating pad
Do not use
- on large areas of the body or on cut, irritated or swollen skin
- on puncture wounds
- for more than one week without consulting a doctor
- Keep out of reach of children.
- Directions
- Other information
-
Inactive ingredients
Acrylates/C10-30 Alkyl Crosspolymer, Aloe Vera Gel 10x, Aminomethyl Propanol, Cetearyl Alcohol, Ceteth-20 Phosphate, Dicetyl Phosphate, Deionized Water, Dimethicone, Caprylyl Methicone, Polysilicone-11, Disodium EDTA, Ethylhexylglycerin, Hydroxyethyl Acrylate/Sodium Acryloyldimethyl Taurate Copolymer, Isohexadecane, Polysorbate 60, Methylparaben, SDA 40-2 Alcohol, Steareth-21
-
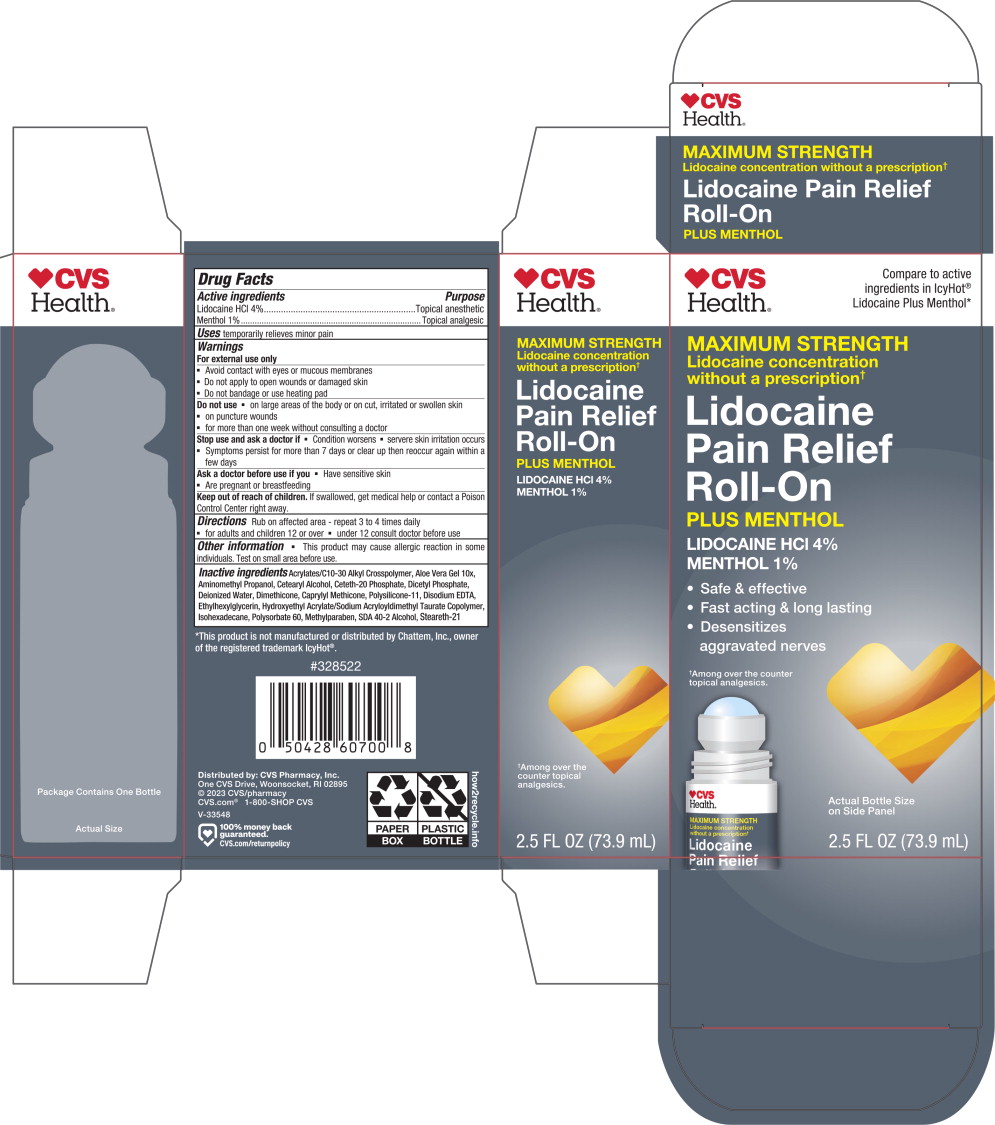
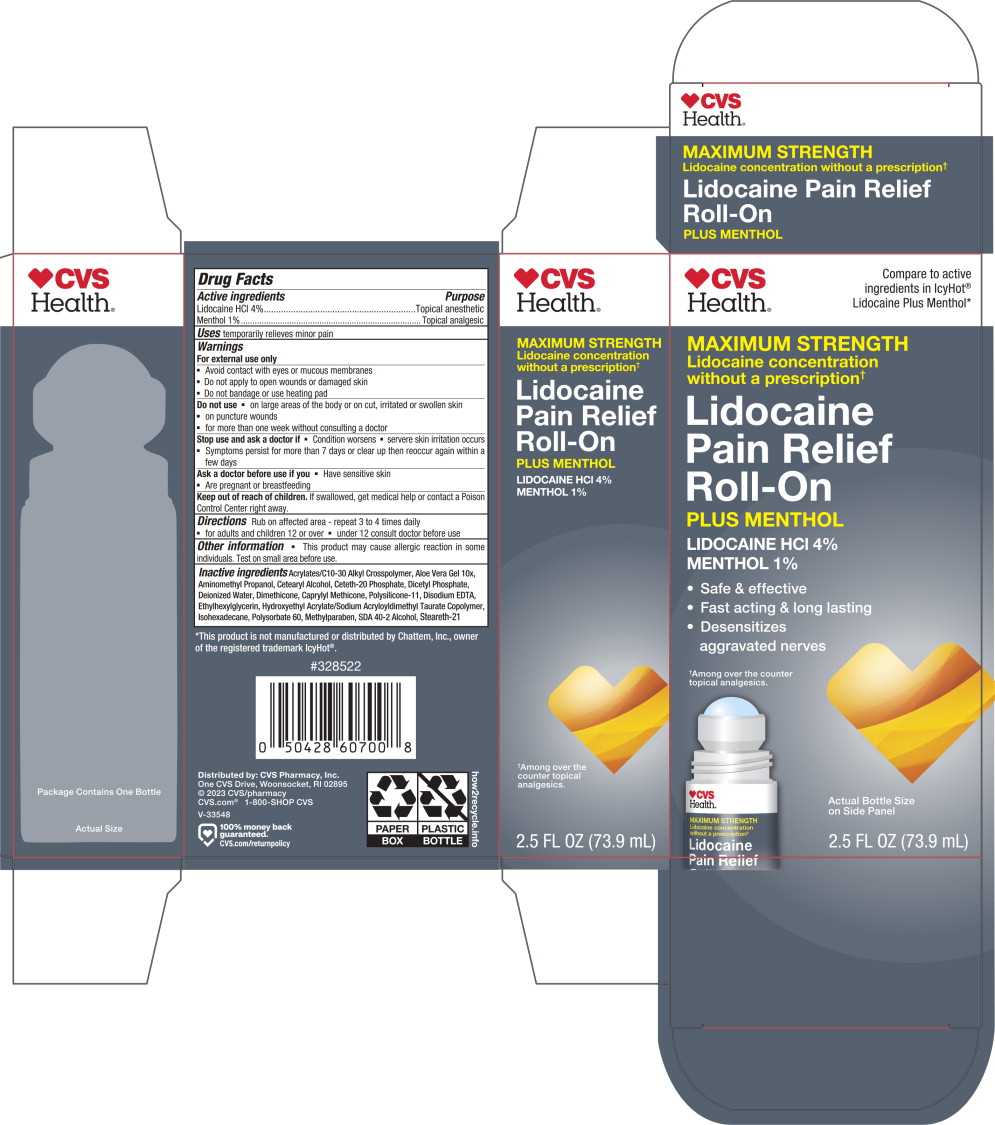
Principal Display Panel – 73.9 mL Carton Label
CVS
Health®Compare to active
ingredients in IcyHot®
Lidocaine Plus Menthol*MAXIMUM STRENGTH
Lidocaine concentration
without a prescription✝Lidocaine
Pain Relief
Roll-OnPLUS MENTHOL
LIDOCAINE HCl 4%
MENTHOL 1%
- Safe & effective
- Fast acting & long lasting
-
Desensitizes
aggravated nerves
✝Among over the counter
topical analgesics.Actual Bottle Size
on Side Panel2.5 FL OZ (73.9 mL)
- Principal Display Panel – 73.9 mL Container Label
-
INGREDIENTS AND APPEARANCE
CVS HEALTH COLD AND HOT ROLL-ON
lidocaine, menthol creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:66902-156 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE (UNII: 98PI200987) (LIDOCAINE - UNII:98PI200987) LIDOCAINE 41 mg in 1 mL MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 9 mg in 1 mL Inactive Ingredients Ingredient Name Strength CARBOMER COPOLYMER TYPE A (UNII: 71DD5V995L) ALOE VERA LEAF (UNII: ZY81Z83H0X) AMINOMETHYLPROPANOL (UNII: LU49E6626Q) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) CETETH-20 PHOSPHATE (UNII: 921FTA1500) DIHEXADECYL PHOSPHATE (UNII: 2V6E5WN99N) WATER (UNII: 059QF0KO0R) DIMETHICONE (UNII: 92RU3N3Y1O) CAPRYLYL TRISILOXANE (UNII: Q95M2P1KJL) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) EDETATE DISODIUM (UNII: 7FLD91C86K) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) HYDROXYETHYL ACRYLATE/SODIUM ACRYLOYLDIMETHYL TAURATE COPOLYMER (100000 MPA.S AT 1.5%) (UNII: 86FQE96TZ4) ISOHEXADECANE (UNII: 918X1OUF1E) POLYSORBATE 60 (UNII: CAL22UVI4M) METHYLPARABEN SODIUM (UNII: CR6K9C2NHK) ALCOHOL (UNII: 3K9958V90M) STEARETH-21 (UNII: 53J3F32P58) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:66902-156-25 1 in 1 BOX 09/14/2018 1 73.9 mL in 1 CONTAINER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 09/14/2018 Labeler - NATURAL ESSENTIALS, INC. (947484713) Establishment Name Address ID/FEI Business Operations NATURAL ESSENTIALS, INC. 947484713 MANUFACTURE(66902-156)