CELLZYME AMPOULE- glycerin, eucalyptus oil, allantoin liquid
PHARMACAL-INTERNATIONAL. CO., LTD
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Cellzyme Ampoule – Ampoule
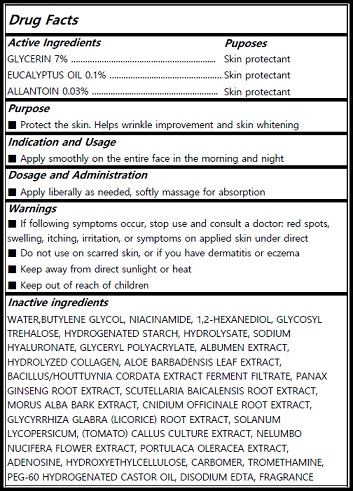
Actvie Ingrdients
GLYCERIN (Skin protectant/7%)
EUCALYPTUS OIL (Skin protectant /0.1%)
ALLANTOIN (Skin protectant/0.03%)
Inactives
WATER,BUTYLENE GLYCOL, NIACINAMIDE, 1,2-HEXANEDIOL, GLYCOSYL TREHALOSE, HYDROGENATED STARCH, HYDROLYSATE, SODIUM HYALURONATE, GLYCERYL POLYACRYLATE, ALBUMEN EXTRACT, HYDROLYZED COLLAGEN, ALOE BARBADENSIS LEAF EXTRACT, BACILLUS/HOUTTUYNIA CORDATA EXTRACT FERMENT FILTRATE, PANAX GINSENG ROOT EXTRACT, SCUTELLARIA BAICALENSIS ROOT EXTRACT, MORUS ALBA BARK EXTRACT, CNIDIUM OFFICINALE ROOT EXTRACT, GLYCYRRHIZA GLABRA (LICORICE) ROOT EXTRACT, SOLANUM LYCOPERSICUM, (TOMATO) CALLUS CULTURE EXTRACT, NELUMBO NUCIFERA FLOWER EXTRACT, PORTULACA OLERACEA EXTRACT, ADENOSINE, HYDROXYETHYLCELLULOSE, CARBOMER, TROMETHAMINE, PEG-60 HYDROGENATED CASTOR OIL, DISODIUM EDTA, FRAGRANCE
| CELLZYME AMPOULE
glycerin, eucalyptus oil, allantoin liquid |
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - PHARMACAL-INTERNATIONAL. CO., LTD (557805060) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| GreenCos Co., Ltd | 694777325 | manufacture(24765-110) | |