CAPXIB- celecoxib, menthol, capsaicin
MAS Management Group
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
INDICATIONS AND USAGE
Carefully consider the potential benefits and risks of celecoxib and other treatment options before deciding to use celecoxib capsules. Use the lowest effective dose for the shortest duration consistent with individual patient treatment goals
DOSAGE AND ADMINISTRATION
Use lowest effective dose for the shortest duration consistent with treatment goals for the individual patient.
These doses can be given without regard to timing of meals.
CONTRAINDICATIONS
Celecoxib capsules are contraindicated:
- In patients with known hypersensitivity to celecoxib, aspirin, or other NSAIDs.
- In patients who have demonstrated allergic-type reactions to sulfonamides.
- In patients who have experienced asthma, urticaria, or allergic-type reactions after taking aspirin or other NSAIDs. Severe anaphylactoid reactions to NSAIDs, some of them fatal, have been reported in such patients.
- For the treatment of peri-operative pain in the setting of coronary artery bypass graft (CABG) surgery
ADVERSE REACTIONS
Of the celecoxib-treated patients in the pre-marketing controlled clinical trials, approximately 4,250 were patients with OA, approximately 2,100 were patients with RA, and approximately 1,050 were patients with post-surgical pain. More than 8,500 patients received a total daily dose of celecoxib of 200 mg (100 mg twice daily or 200 mg once daily) or more, including more than 400 treated at 800 mg (400 mg twice daily). Approximately 3,900 patients received celecoxib at these doses for 6 months or more; approximately 2,300 of these have received it for 1 year or more and 124 of these have received it for 2 years or more.
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice. The adverse reaction information from clinical trials does, however, provide a basis for identifying the adverse events that appear to be related to drug use and for approximating rates.
DRUG INTERACTIONS
General: Celecoxib metabolism is predominantly mediated via cytochrome P450 (CYP) 2C9 in the liver. Co-administration of celecoxib with drugs that are known to inhibit CYP2C9 should be done with caution. Significant interactions may occur when celecoxib is administered together with drugs that inhibit CYP2C9.
In vitro studies indicate that celecoxib, although not a substrate, is an inhibitor of CYP2D6. Therefore, there is a potential for an in vivo drug interaction with drugs that are metabolized by CYP2D6.
OVERDOSAGE
No overdoses of celecoxib were reported during clinical trials. Doses up to 2400 mg/day for up to 10 days in 12 patients did not result in serious toxicity. Symptoms following acute NSAID overdoses are usually limited to lethargy, drowsiness, nausea, vomiting, and epigastric pain, which are generally reversible with supportive care. Gastrointestinal bleeding can occur. Hypertension, acute renal failure, respiratory depression and coma may occur, but are rare. Anaphylactoid reactions have been reported with therapeutic ingestion of NSAIDs, and may occur following an overdose.
Patients should be managed by symptomatic and supportive care following an NSAID overdose. There are no specific antidotes. No information is available regarding the removal of celecoxib by hemodialysis, but based on its high degree of plasma protein binding (>97%) dialysis is unlikely to be useful in overdose. Emesis and/or activated charcoal (60 to 100 g in adults, 1 to 2 g/kg in children) and/or osmotic cathartic may be indicated in patients seen within 4 hours of ingestion with symptoms or following a large overdose. Forced diuresis, alkalinization of urine, hemodialysis, or hemoperfusion may not be useful due to high protein binding.
DESCRIPTION
Celecoxib is chemically designated as 4-[5-(4-methylphenyl)- 3-(trifluoromethyl)-1H-pyrazol-1-yl] benzenesulfonamide and is a diaryl-substituted pyrazole. The empirical formula is C17H14F3N3O2S, and the molecular weight is 381.38; the chemical structure is as follows:
Celecoxib capsules for oral administration contain either 50 mg, 100 mg, or 200 mg of celecoxib, together with inactive ingredients including: crospovidone, sodium lauryl sulphate, povidone, magnesium stearate. The capsule shell contains gelatin and titanium dioxide. The capsule imprinting ink for the 50 mg strength contains shellac, ethyl alcohol, isopropyl alcohol, butyl alcohol, propylene glycol, sodium hydroxide, titanium dioxide, povidone and FD&C Red #40 Aluminum Lake. The capsule imprinting ink for the 100 mg strength contains shellac, ethyl alcohol, isopropyl alcohol, butyl alcohol, propylene glycol, strong ammonia solution, and FD&C Blue #2 Aluminum Lake. The capsule imprinting ink for the 200 mg strength contains shellac, ethyl alcohol, isopropyl alcohol, butyl alcohol, propylene glycol, strong ammonia solution, iron oxide yellow, and dimethicone.
HOW SUPPLIED/STORAGE AND HANDLING
Celecoxib capsules 50 mg are available for oral administration as hard gelatin capsules with a white opaque body and a white opaque cap. “APO C50” is imprinted on each capsule in red ink. They are supplied as follows:
Bottles of 30s NDC 60505-3847-3
Bottles of 60s NDC 60505-3847-6
Bottles of 100s NDC 60505-3847-1
Bottles of 1000 NDC 60505-3847-8
Unit Dose Blisters of 100 NDC 60505-3847-0
Celecoxib capsules 100 mg are available for oral administration as hard gelatin capsules with a white opaque body and a white opaque cap. “APO C100” is imprinted on each capsule in blue ink. They are supplied as follows:
Bottles of 30s NDC 60505-3848-3
Bottles of 100s NDC 60505-3848-1
Bottles of 500s NDC 60505-3848-5
Bottles of 1000 NDC 60505-3848-8
Unit Dose Blisters of 100 NDC 60505-3848-0
Celecoxib capsules 200 mg are available for oral administration as hard gelatin capsules with a white opaque body and a white opaque cap. “APO C200” is imprinted on each capsule in yellow ink. They are supplied as follows:
Bottles of 30s NDC 60505-3849-3
Bottles of 100s NDC 60505-3849-1
Bottles of 500s NDC 60505-3849-5
Bottles of 1000 NDC 60505-3849-8
Unit Dose Blisters of 100 NDC 60505-3849-0
PATIENT COUNSELING INFORMATION
Patients should be informed of the following information before initiating therapy with celecoxib capsules and periodically during the course of ongoing therapy.
MEDICATION GUIDE
Medication Guide for
Nonsteroidal Anti-Inflammatory Drugs (NSAIDs)
What is the most important information I should know about medicines called Nonsteroidal Anti-Inflammatory Drugs (NSAIDs)?
NSAIDs can cause serious side effects, including:
Increased risk of a heart attack or stroke that can lead to death. This risk may happen early in treatment and may increase:
- with increasing doses of NSAIDs
- with longer use of NSAIDs
Do not take NSAIDs right before or after a heart surgery called a “coronary artery bypass graft (CABG)."
Avoid taking NSAIDs after a recent heart attack, unless your healthcare provider tells you to. You may have an increased risk of another heart attack if you take NSAIDs after a recent heart attack.
Increased risk of bleeding, ulcers, and tears (perforation) of the esophagus (tube leading from the mouth to the stomach), stomach and intestines:
- anytime during use
- without warning symptoms
- that may cause death
The risk of getting an ulcer or bleeding increases with:
- past history of stomach ulcers, or stomach or intestinal bleeding with use of NSAIDs
- taking medicines called “corticosteroids”, “anticoagulants”, “SSRIs”, or “SNRIs”
- increasing doses of NSAIDs
- longer use of NSAIDs
- smoking
- drinking alcohol
- older age
- poor health
- advanced liver disease
- bleeding problems
NSAIDs should only be used: :
- exactly as prescribed
- at the lowest dose possible for your treatment
- for the shortest time needed
What are NSAIDs?
NSAIDs are used to treat pain and redness, swelling, and heat (inflammation) from medical conditions such as different types of arthritis, menstrual cramps, and other types of short-term pain.
Who should not take NSAIDs?
Do not take NSAIDs:
- if you have had an asthma attack, hives, or other allergic reaction with aspirin or any other NSAIDs.
- right before or after heart bypass surgery.
Before taking NSAIDs, tell your healthcare provider about all of your medical conditions, including if you:
- have liver or kidney problems
- have high blood pressure
- have asthma
- are pregnant or plan to become pregnant. Talk to your healthcare provider if you are considering taking NSAIDs during pregnancy. You should not take NSAIDs after 29 weeks of pregnancy.
- are breastfeeding or plan to breast feed.
Tell your healthcare provider about all of the medicines you take, including prescription or over-the-counter medicines, vitamins or herbal supplements. NSAIDs and some other medicines can interact with each other and cause serious side effects. Do not start taking any new medicine without talking to your healthcare provider first.
What are the possible side effects of NSAIDs?
NSAIDs can cause serious side effects, including:
See “What is the most important information I should know about medicines called Nonsteroidal Anti-inflammatory Drugs (NSAIDs)?
- new or worse high blood pressure
- heart failure
- liver problems including liver failure
- kidney problems including kidney failure
- low red blood cells (anemia)
- life-threatening skin reactions
- life-threatening allergic reactions
Other side effects of NSAIDs include: stomach pain, constipation, diarrhea, gas, heartburn, nausea, vomiting, and dizziness.
Get emergency help right away if you get any of the following symptoms:
- shortness of breath or trouble breathing
- chest pain
- weakness in one part or side of your body
- slurred speech
- swelling of the face or throat
Stop taking your NSAID and call your healthcare provider right away if you get any of the following symptoms:
- nausea
- more tired or weaker than usual
- diarrhea
- itching
- your skin or eyes look yellow
- indigestion or stomach pain
- flu-like symptoms
- vomit blood
- there is blood in your bowel movement or it is black and sticky like tar
- unusual weight gain
- skin rash or blisters with fever
- swelling of the arms, legs, hands and feet
If you take too much of your NSAID, call your healthcare provider or get medical help right away.
These are not all the possible side effects of NSAIDs. For more information, ask your healthcare provider or pharmacist about NSAIDs.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
Other information about NSAIDs
- Aspirin is an NSAID but it does not increase the chance of a heart attack. Aspirin can cause bleeding in the brain, stomach, and intestines. Aspirin can also cause ulcers in the stomach and intestines.
- Some NSAIDs are sold in lower doses without a prescription (over-the-counter). Talk to your healthcare provider before using over-the-counter NSAIDs for more than 10 days.
General information about the safe and effective use of NSAIDs
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use NSAIDs for a condition for which it was not prescribed. Do not give NSAIDs to other people, even if they have the same symptoms that you have. It may harm them.
If you would like more information about NSAIDs, talk with your healthcare provider. You can ask your pharmacist or healthcare provider for information about NSAIDs that is written for health professionals.
This Medication Guide has been approved by the U.S. Food and Drug Administration.
APOTEX INC.
CELECOXIB CAPSULES
50 mg, 100 mg and 200 mg
Manufactured by: Manufactured for:
Apotex Inc Apotex Corp.
Toronto, Ontario Weston, Florida
Canada, M9L 1T9USA 33326
Revised: July 2015
USES
For the temporary relief of minor aches and pains of the muscles and joints associated with simple backache, arthritis, sprains, brulses and strains.
WARNINGS
- For external use only. Use only as directed. Avoid contact with eyes and mucous membranes.
- Do not cover with bandage.
- Do not use on wounds or damaged skin.
- Do not use in combination with any other external analgesic products.
- Avoid Contact with eyes and mucous membranes.
- Do not use on irritated or broken skin, or use in combination with any bandage, wrap, stocking or similar device or garment.
OTHER INGREDIENTS
WATER, GLYCERINE, SODIUM POLYACRYLATE, POLYSORBATE 80, ALOE BARBADESIS LEAF (ALOE VERA
GEL) JUICE, EDTA DISODIUM SALT, DIAZOLIDINYL UREA, METHYLPARABEN, LODOPROYNYL BUTYLCABAMATE, PROPYLPARABEN.
| CAPXIB
celecoxib, menthol, capsaicin kit |
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
| Labeler - MAS Management Group (079363782) |


 NDC # 69677-040-02
NDC # 69677-040-02
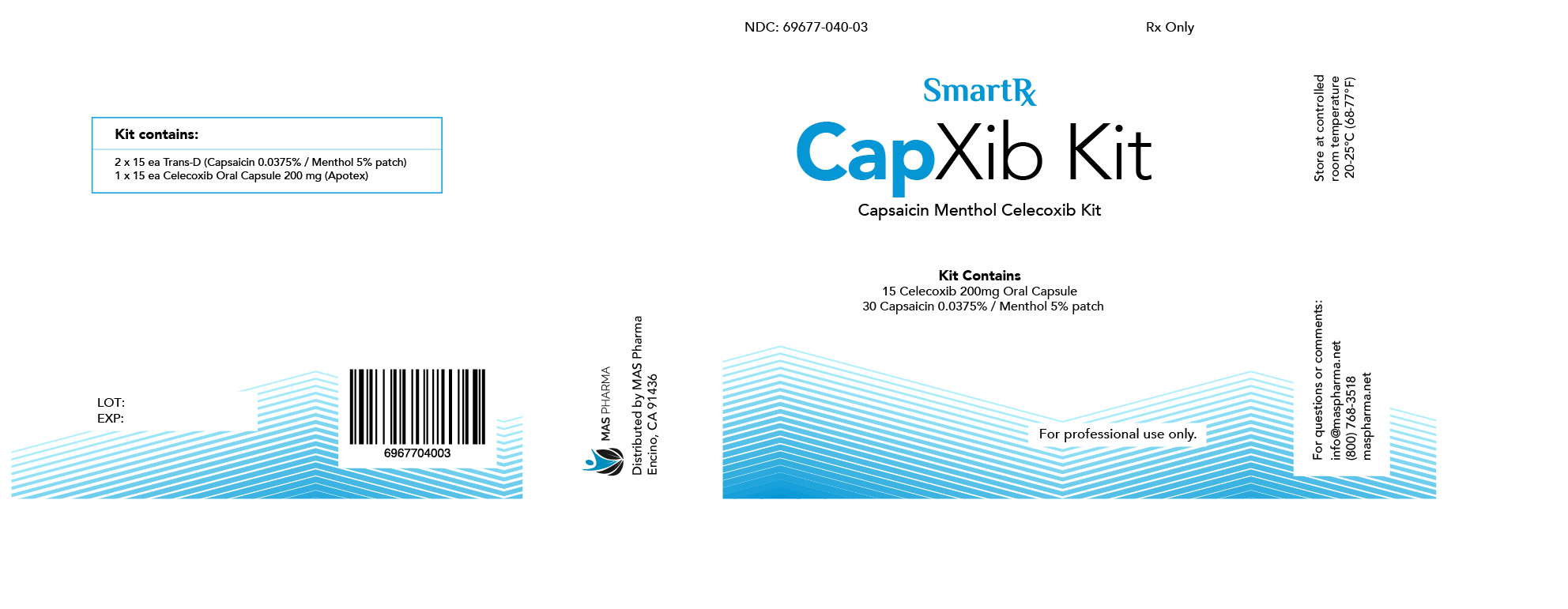 NDC # 69677-040-03
NDC # 69677-040-03