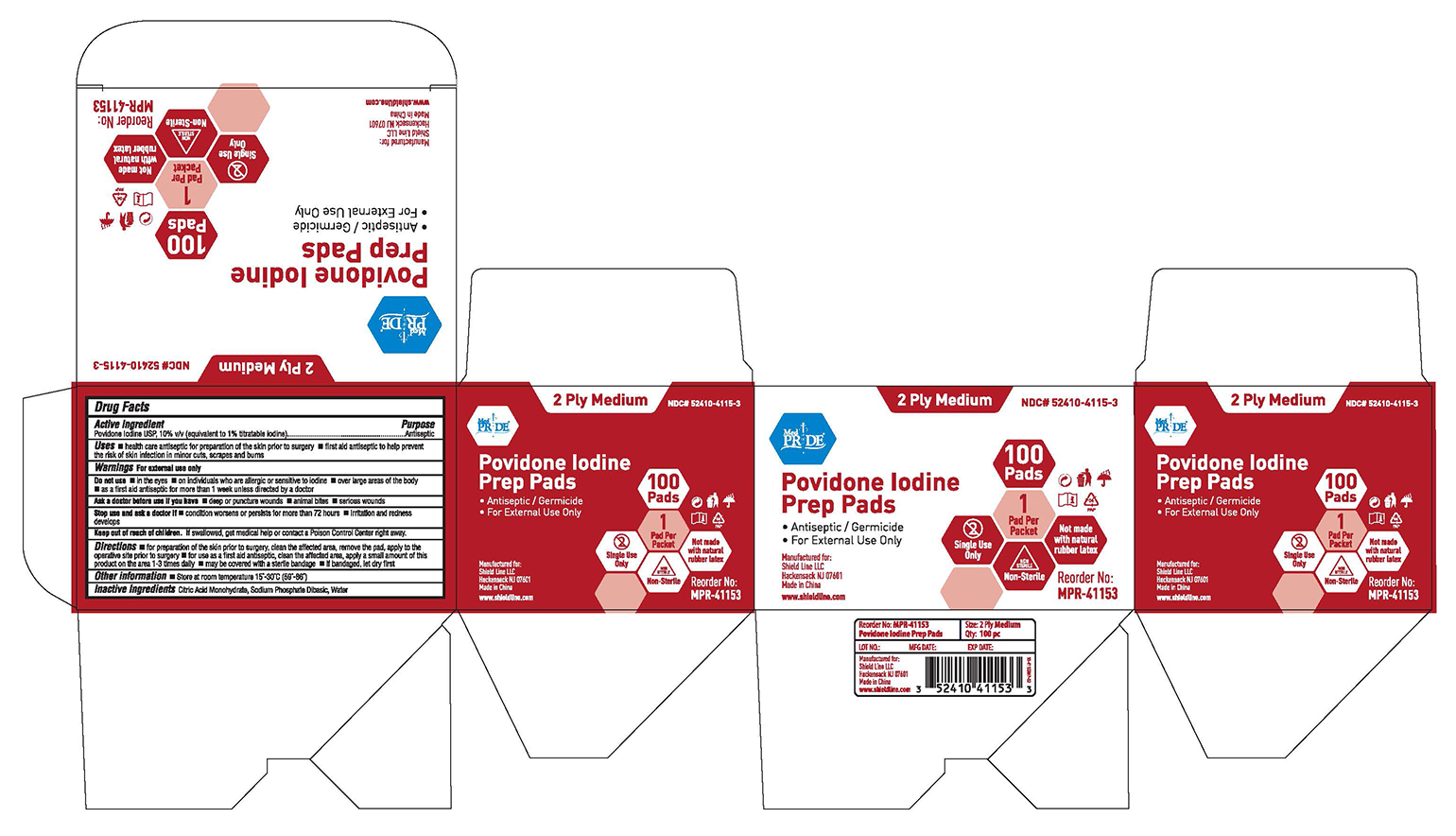
Label: MEDPRIDE PREPPADS- povidone iodine solution
- NDC Code(s): 52410-4115-3
- Packager: Shield Line LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 14, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
- Uses
- Warnings
- Do not use
- Ask a doctor before use if you have
- Stop use and ask a doctor if
- Keep out of reach of children
-
Directions
■ for preparation of the skin prior to surgery, clean the affected area, remove the pad, apply to the operative site prior to surgery
■ for use as a first aid antiseptic, clean the affected area, apply a small amount of this product on the area 1-3 times daily ■ may be covered with a sterile bandage ■ if bandaged, let dry first
- Other Information
- Inactive Ingredients
- Package Label
-
INGREDIENTS AND APPEARANCE
MEDPRIDE PREPPADS
povidone iodine solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:52410-4115 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength POVIDONE-IODINE (UNII: 85H0HZU99M) (IODINE - UNII:9679TC07X4) IODINE 0.1 g Inactive Ingredients Ingredient Name Strength CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) WATER (UNII: 059QF0KO0R) SODIUM PHOSPHATE, DIBASIC (UNII: GR686LBA74) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:52410-4115-3 100 in 1 BOX; Type 0: Not a Combination Product 10/20/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M003 10/20/2018 Labeler - Shield Line LLC (078518916)