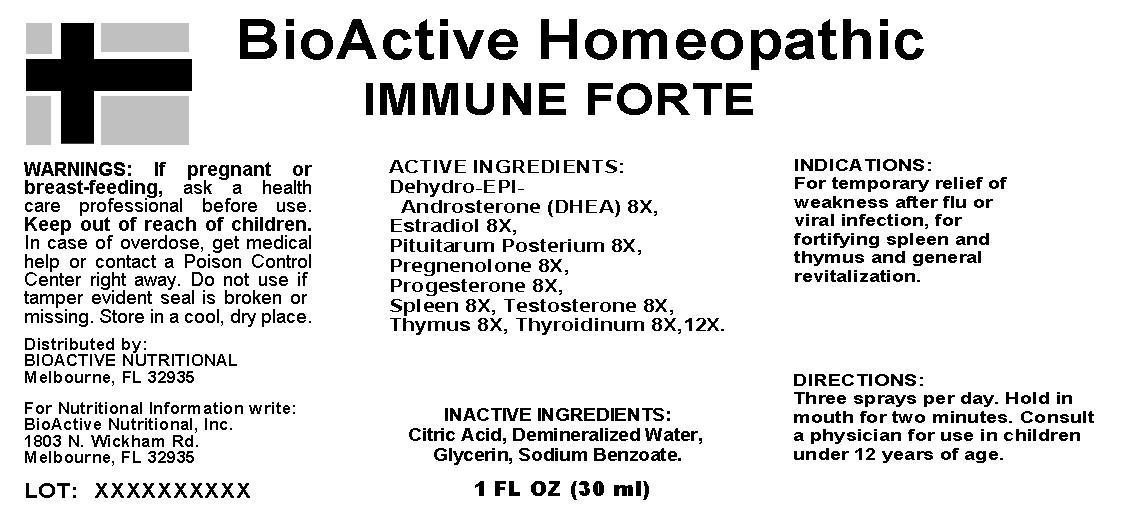
IMMUNE FORTE- dehydro-epi-androsterone(dhea), estradiol, pituitarum posterium, pregnenolone, progesterone, spleen, testosterone, thymus, thyroidinum, spray
Apotheca Company
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
Immune Forte
Active Ingredients: Dehydro-Epi-Androsterone (DHEA),Estradiol 8X, Pituitarum Posterium, Pregnenolone 8X, Progesterone 8X, Spleen 8X, Testosterone 8X, Thymus 8X, Thyroidinum 8X, 12X.
Indications: For temporary relief of weakness after flu or viral infection, for fortifying spleen and thymus and general revitalization.
WARNINGS: If pregnant or breast-feeding, ask a health care professional before use.
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
Do not use if tamper evident seal is broken or missing.
Store in a cool, dry place.
KEEP OUT OF REACH OF CHILDREN:
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
Directions: Three sprays per day. Hold in mouth for two minutes. Consult a physician for use in children under 12 years of age.
INDICATIONS:
For temporary relief of weakness after flu or viral infection, for fortifying spleen and thymus and general revitalization.
| IMMUNE FORTE
dehydro-epi-androsterone(dhea), estradiol, pituitarum posterium, pregnenolone, progesterone, spleen, testosterone, thymus, thyroidinum, spray |
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
| Labeler - Apotheca Company (844330915) |
| Registrant - Apotheca Company (844330915) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Apotheca Company | 844330915 | manufacture(57520-0120) , api manufacture(57520-0120) , label(57520-0120) , pack(57520-0120) | |