SULFACETAMIDE SODIUM - sulfacetamide sodium ointment
Fera Pharmaceuticals, LLC
----------
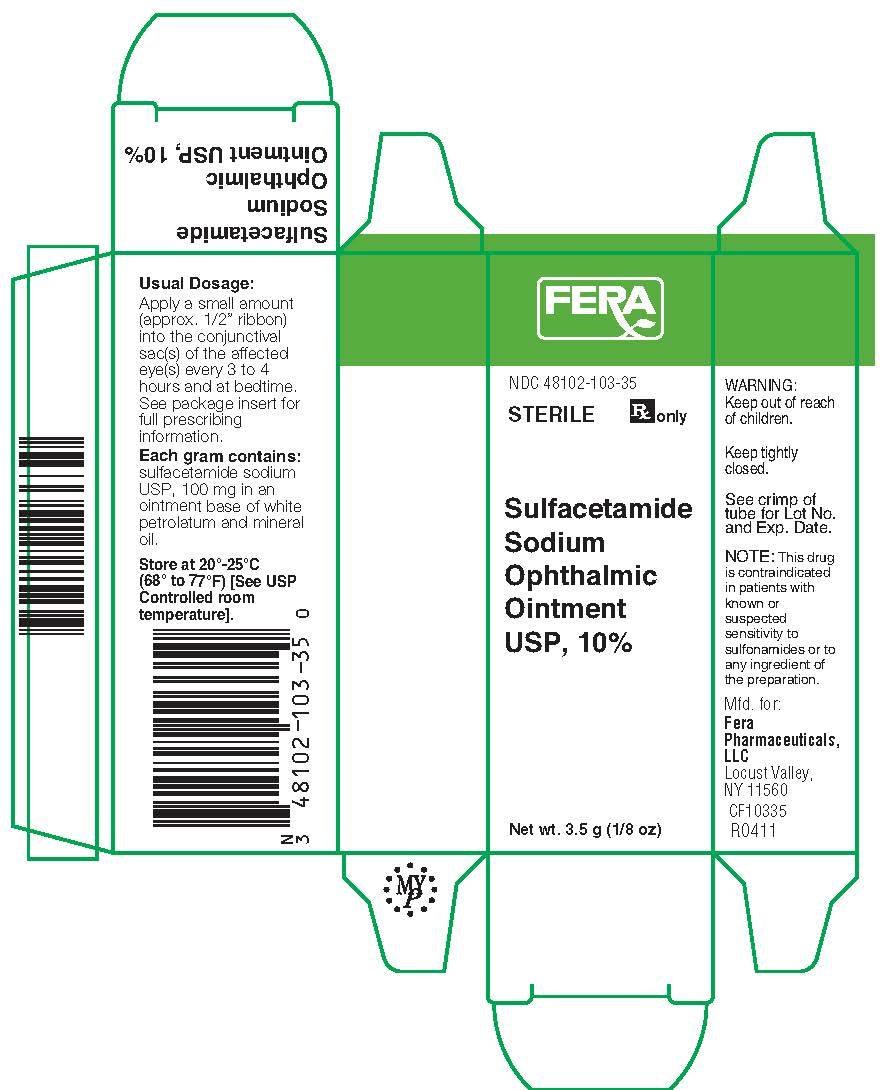
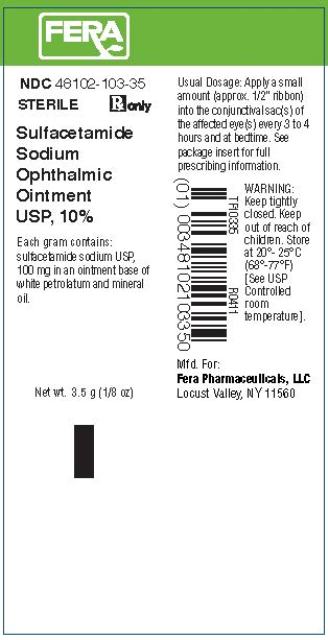
SULFACETAMIDE SODIUM OPHTHALMIC OINTMENT USP, 10% Sterile Rx Only
DESCRIPTION:
Sulfacetamide Sodium Ointment USP, 10%, is a sterile, topical anti-bacterial agent for ophthalmic use. Each gram contains Sulfacetamide Sodium USP, 100 mg in an ointment base of white petrolatum and mineral oil.
Sulfacetamide sodium is an odorless, white, crystalline powder. It is freely soluable in water, sparingly soluble in alcohol, and practically insoluble in benzene, chloroform, and ether. Chemically it is N-sulfanilylacetamide monosodium salt monohydrate, and is represented by the following structural formula:
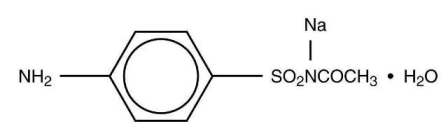
Molecular Formula: C8H9N2NaO3S•H2O
Molecular Weight 254.24
CLINICAL PHARMACOLOGY:
Microbiology:
The sulfonamides are bacteriostatic agents and the spectrum of activity is similar for all. Sulfonamides inhibit bacterial synthesis of dihydrofolic acid by preventing the condensation of pteridine with aminobenzoic acid through competitive inhibition of the enzyme dihydropteroate synthetase. Resistant strains have altered dihydropteroate synthetase with reduced affinity for sulfonamides or produce increased quantities of aminobenzoic acid.
Topically applied sulfonamides are considered active against susceptible strains of the following common bacterial eye pathogens: Escherichia coli, Staphylococcus aureus,Streptococcus pneumoniae, Streptococcus (viridans group), Haemophilus influenzae,Klebsiella species, and Enterobacter species.
Topically applied sulfonamides do not provide adequate coverage against Neisseria species, Serratia marcescens and Pseudomonas aeroginosa. A significant percentage of staphylococcal isolates are completely resistant to sulfa drugs.
INDICATIONS AND USAGE:
For the treatment of conjunctivitis and other superficial ocular infections due to susceptible microorganisms:
Escherichia coli, Staphylococcus aureus, Streptococcus pneumoniae, Streptococcus (viridans group), Haemophilus influenzae, Klebsiella species, and Enterobacter species.
Topically applied sulfonamides do not provide adequate coverage against Neisseria species, Serratia marcescens and Pseudomonas aeroginosa. A significant percentage of staphylococcal isolates are completely resistant to sulfa drugs.
WARNINGS:
FOR TOPICAL EYE USE ONLY-NOT FOR INJECTION.
FATALITIES HAVE OCCURRED, ALTHOUGH RARELY, DUE TO SEVERE REACTIONS TO SULFONAMIDES INCLUDING STEVENS-JOHNSON SYNDROME, TOXIC EPIDERMAL NECROLYSIS, FULMINANT HEPATIC NECROSIS, AGRANULOCYTOSIS, APLASTIC ANEMIA AND OTHER BLOOD DYSCRASIAS. Sensitizations may recur when a sulfonamide is readministered, irrespective of the route of administration. Sensitivity reactions have been reported in individuals with no prior history of sulfonamide hypersensitivity. At the first sign of hypersensitivity, skin rash or other serious reaction, discontinue use of this preparation.
PRECAUTIONS
General:
Prolonged use of topical anti-bacterial agents may give rise to overgrowth of nonsusceptible organisms including fungi. Bacterial resistance to sulfonamides may also develop.
Ophthalmic ointments may retard corneal wound healing.
The effectiveness of sulfonamides may be reduced by the para-aminobenzoic acid present in the purulent exudates. Sensitization may recur when a sulfonamide is readministered irrespective of the route of administration, and cross-sensitivity between different sulfonamides may occur.
At the first sign of hypersensitivity, increase in purulent discharge, or aggravation of inflammation or pain, the patient should discontinue use of the medication and consult a physician (see WARNINGS).
Information for patients:
To avoid contamination, do not touch tip of container to eye, eyelid, or any surface.
Carcinogenesis, Mutagenesis, Impairment of Fertility:
No studies have been conducted in animals or in humans to evaluate the possibility of these effects with ocularly administered sulfacetamide. Rats appear to be especially susceptible to the goitrogenic effects of sulfonamides, and long-term oral administration of sulfonamides has resulted in thyroid malignancies in these animals.
Pregnancy:
Teratogenic effects. Pregnancy Category C. Animal reproduction studies have not been conducted with sulfonamide ophthalmic preparations. Kernicterus may occur in the newborn as a result of treatment of a pregnant woman at term with orally administered sulfonamides. There are no adequate and well controlled studies of sulfonamide ophthalmic preparations in pregnant women and it is not known whether topically applied sulfonamides can cause fetal harm when administered to a pregnant woman. This product should be used in pregnancy only if the potential benefit justifies the potential risk to the fetus.
Nursing Mothers:
Systemically administered sulfonamides are capable of producing kernicterus in infants of lactating women. Because of the potential for the development of kernicterus in neonates, a decision should be made whether to discontinue nursing or discontinue the drug taking into account the importance of the drug to the mother.
Pediatric Use:
Safety and effectiveness in children below the age of two months have not been established.
ADVERSE REACTIONS:
Bacterial and fungal corneal ulcers have been developed during treatment with sulfonamide ophthalmic preparations.
The
most frequently reported reactions are local irritation, stinging and burning. Less commonly reported reactions include non-specific conjunctivitis,
conjunctival hyperemia, secondary infections and allergic reactions. Fatalities have occurred, although rarely, due to severe reactions to sulfonamides including Stevens-Johnson syndrome, toxic epidermal necrolysis, fulminant hepatic necrosis, agranulocytosis, aplastic anemia, and other blood dyscrasias (see WARNINGS).
To report SUSPECTED ADVERSE REACTIONS, contact Fera Pharmaceuticals, LLC at (414) 434-6604, Monday - Friday 9am-5pm EST, or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DOSAGE AND ADMINISTRATION:
For conjunctivitis and other superficial ocular infections: Apply a small amount (approximately one-half inch ribbon) into the conjunctival sac(s) of the affected eye(s) every three to four hours and at bedtime. Dosages may be tapered by increasing the time interval between doses as the condition responds. The ointment may be used as adjunct to the solution. The usual duration of treatment is seven to ten days.
HOW SUPPLIED:
Sulfacetamide Sodium Ophthalmic Ointment USP, 10%, is supplied in 3.5 gram (1/8 Oz) sterile, tamper evident tubes, NDC 48102-103-35.
Store at 20o-25oC (68o-77oF) [See USP Controlled room temperature].
FERA
Mfd. for:
Fera Pharmaceuticals, LLC
Locust Valley, NY 11560
PF10335A
R0212
| SULFACETAMIDE SODIUM
sulfacetamide sodium ointment |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Fera Pharmaceuticals, LLC (831023713) |
| Registrant - Fera Pharmaceuticals, LLC (831023713) |