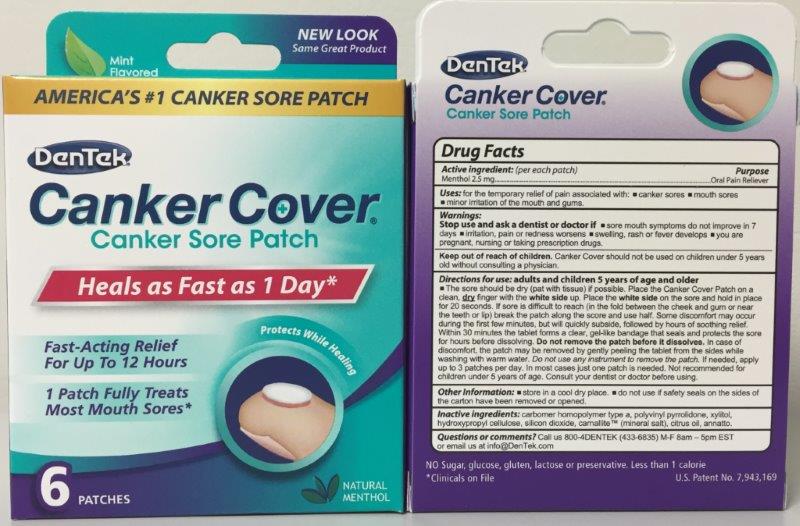
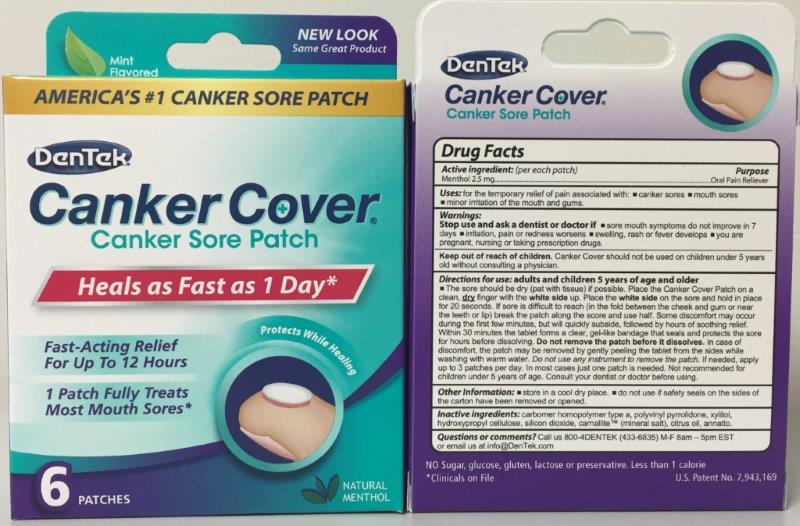
Label: DENTEK CANKER COVER- menthol patch, extended release
-
Contains inactivated NDC Code(s)
NDC Code(s): 10564-0910-2 - Packager: Bershtel Enterprises
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated December 4, 2015
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Keep Out of Reach of Children Section
- Purpose Section
- Ask Doctor
-
Indications & Usage
Adult and Children 5 year of age and older.
The sore should be dry (pat with tissue) is possible. Place the Canker Cover Patch on a clean, dry finger with the white side up. Place the white side on the sore and hold in place for 20 seconds. Within 30 minutes the tablet forms a clear, gel-like bandage that seals and protects the sore for hours before disolving. DO NOT REMOVE PATCH BEFORE IT DISSOLVES.
- Dosage & Administration
- Questions section
- Warning Section
- Inactive Ingredients
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
DENTEK CANKER COVER
menthol patch, extended releaseProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:10564-0910 Route of Administration TRANSMUCOSAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 2.5 mg in 194.85 Inactive Ingredients Ingredient Name Strength CITRUS MEDICA FRUIT (UNII: ZE5Q6PN9ON) CARBOMER 934 (UNII: Z135WT9208) POVIDONE (UNII: FZ989GH94E) HYDROXYPROPYL CELLULOSE (TYPE E) (UNII: 66O7AQV0RT) SEA SALT (UNII: 87GE52P74G) XYLITOL (UNII: VCQ006KQ1E) MAGNESIUM STEARATE (UNII: 70097M6I30) Product Characteristics Color Score Shape ROUND Size 9mm Flavor MENTHOL Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:10564-0910-2 1 in 1 CARTON; Type 0: Not a Combination Product 12/01/2015 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part356 12/01/2015 Labeler - Bershtel Enterprises (066659129)