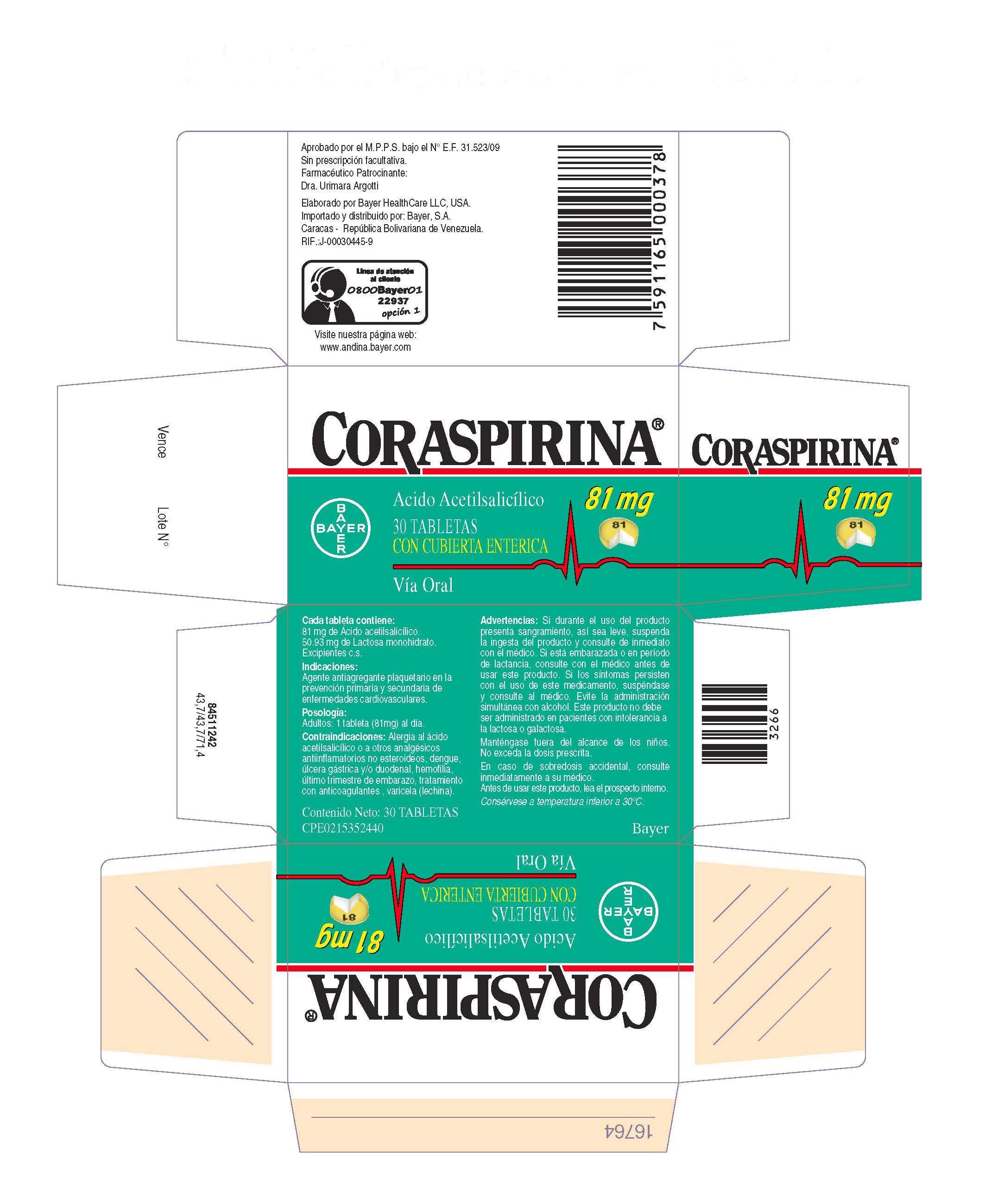
Label: CORASPIRINA 81 MG TABLETAS CON CUBIERTA ENTERICA- coraspirin 81 mg enteric coasted tablet tablet, coated
- NDC Code(s): 0280-2055-30
- Packager: Bayer HealthCare LLC.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Export only
Drug Label Information
Updated January 10, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
CORASPIRINA 81 MG TABLETAS CON CUBIERTA ENTERICA
coraspirin 81 mg enteric coasted tablet tablet, coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0280-2055 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ASPIRIN (UNII: R16CO5Y76E) (ASPIRIN - UNII:R16CO5Y76E) ASPIRIN 81 mg Inactive Ingredients Ingredient Name Strength BROWN IRON OXIDE (UNII: 1N032N7MFO) POWDERED CELLULOSE (UNII: SMD1X3XO9M) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) STARCH, CORN (UNII: O8232NY3SJ) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) HYPROMELLOSES (UNII: 3NXW29V3WO) METHACRYLIC ACID - ETHYL ACRYLATE COPOLYMER (1:1) TYPE A (UNII: NX76LV5T8J) POLYSORBATE 80 (UNII: 6OZP39ZG8H) ALUMINUM OXIDE (UNII: LMI26O6933) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) SODIUM LAURYL SULFATE (UNII: 368GB5141J) FERROSOFERRIC OXIDE (UNII: XM0M87F357) CARNAUBA WAX (UNII: R12CBM0EIZ) SHELLAC (UNII: 46N107B71O) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) TRIACETIN (UNII: XHX3C3X673) TRIETHYL CITRATE (UNII: 8Z96QXD6UM) Product Characteristics Color yellow Score no score Shape ROUND (convex discoid) Size 5mm Flavor Imprint Code 81 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0280-2055-30 1 in 1 CARTON 08/01/2001 1 30 in 1 BOTTLE; Type 0: Not a Combination Product 
Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Export only 08/01/2001 Labeler - Bayer HealthCare LLC. (112117283)