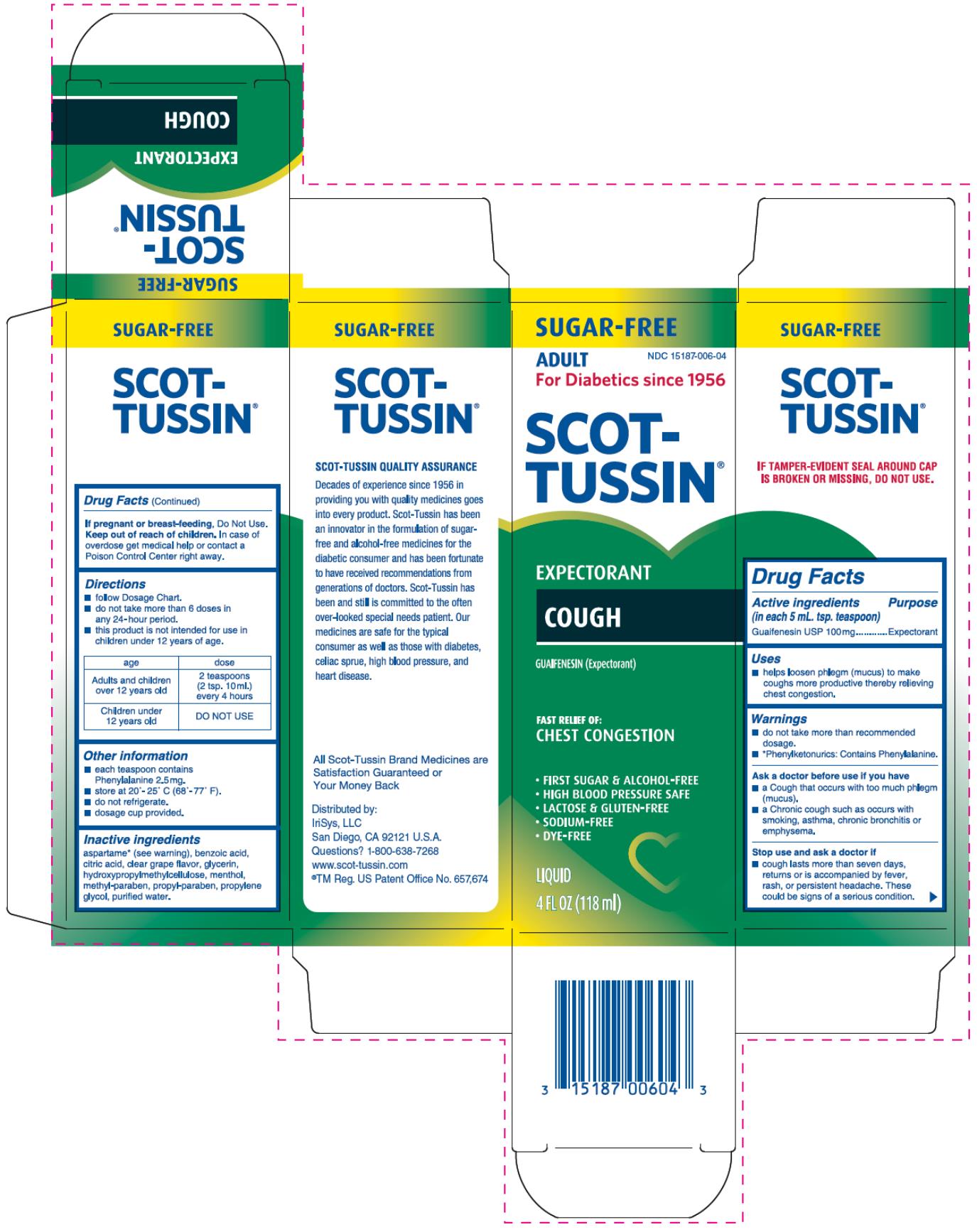
SCOT-TUSSIN EXPECTORANT COUGH- guaifenesin liquid
SOCIETAL CDMO SAN DIEGO, LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Scot-Tussin Expectorant Cough
Warnings
- do not take more than the recommended dosage.
- *Phenylketoneurics: Contains Phenylalanine.
Ask a doctor before use if you have
- a Cough that occurs with too much phlegm (mucus).
- a Chronic cough such as occurs with smoking, asthma, chronic bronchitis or emphysema.
Directions
- follow Dosage Chart.
- do not take more than 6 doses in any 24-hour period.
- this product is not intended for use in children under 12 years of age.
| age | dose |
| Adults and children over 12 years old | 2 teaspoons (2 tsp. 10 mL) every 4 hours |
| Children under 12 years old | DO NOT USE |
Other information
- each teaspoon contains Phenylalanine 2.5 mg.
- store at 20° - 25° C (68° - 77° F).
- do not refrigerate.
- dosage cup provided.
Inactive ingredients
aspartame* (see warning), benzoic acid, citric acid, clear grape flavor, glycerin, hydroxypropylmethylcellulose, menthol, methyl-paraben, propyl-paraben, propylene glycol, purified water.
Distributed by:
IriSys, LLC
San Diego, CA 92121 U.S.A.
Questions? 1-800-638-7268
www.scot-tussin.com
®TM Reg. US Patent Office No. 657, 674
| SCOT-TUSSIN EXPECTORANT COUGH
guaifenesin liquid |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - SOCIETAL CDMO SAN DIEGO, LLC (079682716) |
Revised: 5/2023
Document Id: fc109f3d-9f3f-4ee1-e053-6294a90a30ac
Set id: 250dac24-75a4-454c-9069-a9e52ab763f5
Version: 8
Effective Time: 20230519
SOCIETAL CDMO SAN DIEGO, LLC