VITALUMIERE MOISTURE-RICH RADIANCE SUNSCREEN FLUID MAKEUP, BROAD SPECTRUM SPF 15, 20 CLAIR- octinoxate emulsion
VITALUMIERE MOISTURE-RICH RADIANCE SUNSCREEN FLUID MAKEUP, BROAD SPECTRUM SPF 15, 30 CENDRE- octinoxate emulsion
VITALUMIERE MOISTURE-RICH RADIANCE SUNSCREEN FLUID MAKEUP, BROAD SPECTRUM SPF 15, 40 BEIGE- octinoxate emulsion
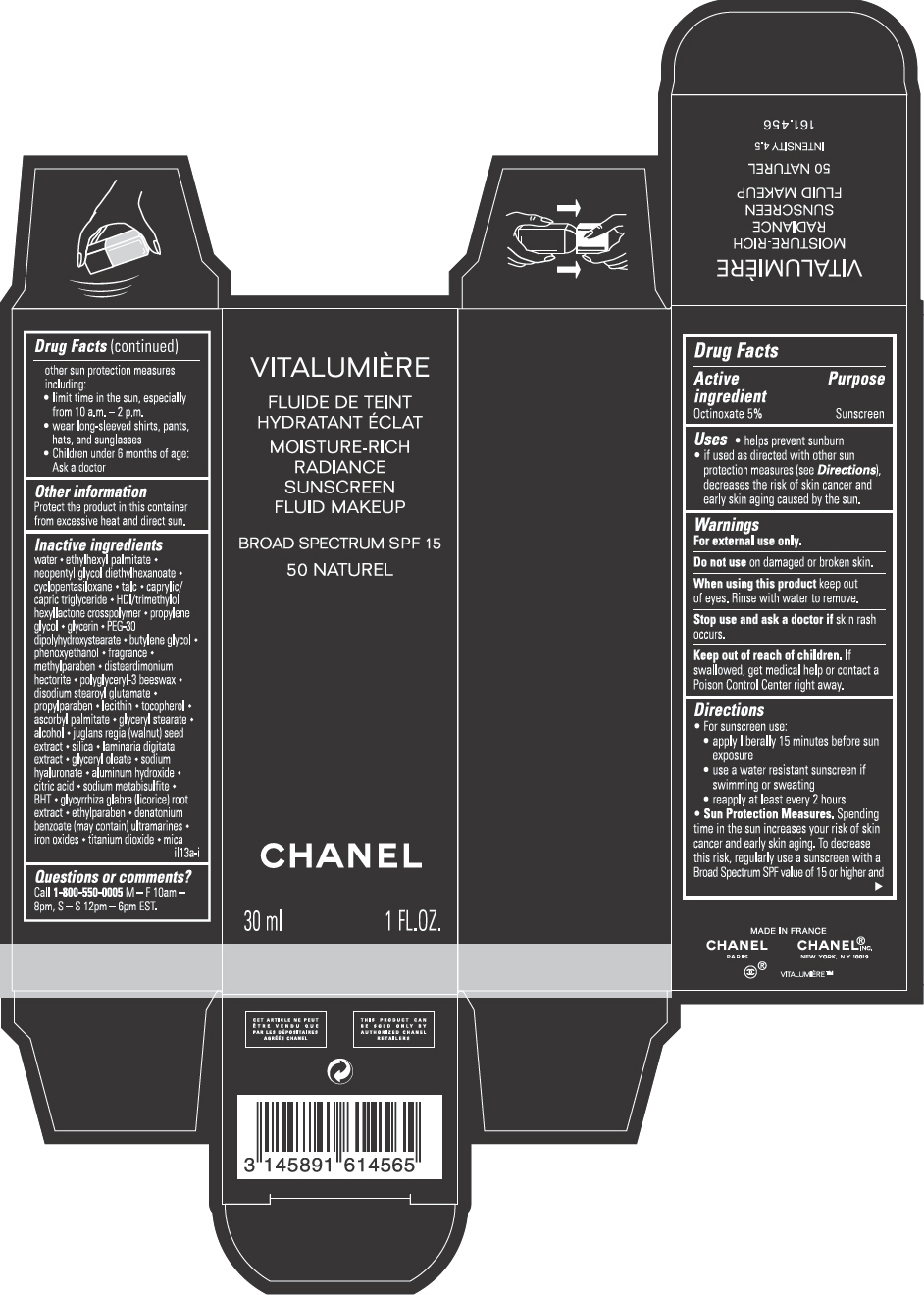
VITALUMIERE MOISTURE-RICH RADIANCE SUNSCREEN FLUID MAKEUP, BROAD SPECTRUM SPF 15, 50 NATUREL- octinoxate emulsion
VITALUMIERE MOISTURE-RICH RADIANCE SUNSCREEN FLUID MAKEUP, BROAD SPECTRUM SPF 15, 07 IVOIRE- octinoxate emulsion
VITALUMIERE MOISTURE-RICH RADIANCE SUNSCREEN FLUID MAKEUP, BROAD SPECTRUM SPF 15, 35 SOFT BISQUE- octinoxate emulsion
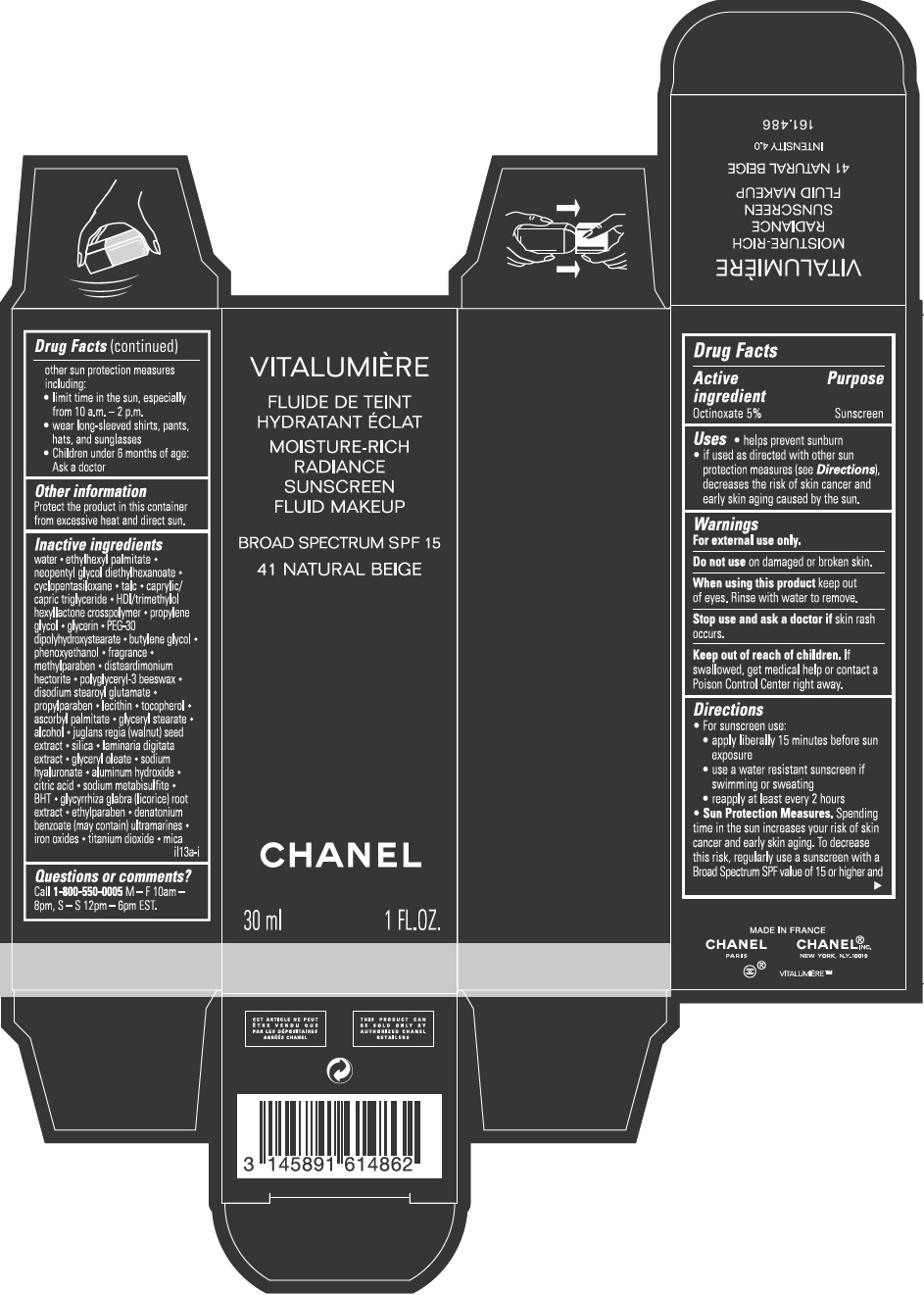
VITALUMIERE MOISTURE-RICH RADIANCE SUNSCREEN FLUID MAKEUP, BROAD SPECTRUM SPF 15, 41 NATURAL BEIGE- octinoxate emulsion
VITALUMIERE MOISTURE-RICH RADIANCE SUNSCREEN FLUID MAKEUP, BROAD SPECTRUM SPF 15, 51 TAWNY BEIGE- octinoxate emulsion
CHANEL PARFUMS BEAUTE
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active ingredient
Octinoxate 5%
Uses
- helps prevent sunburn
- if used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun.
Warnings
For external use only.
Do not use on damaged or broken skin.
When using this product keep out of eyes. Rinse with water to remove.
Stop use and ask a doctor if skin rash occurs.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- For sunscreen use:
- apply liberally 15 minutes before sun exposure
- use a water resistant sunscreen if swimming or sweating
- reapply at least every 2 hours
-
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m. – 2 p.m.
- wear long-sleeved shirts, pants, hats, and sunglasses
- Children under 6 months of age: Ask a doctor
Other information
Protect the product in this container from excessive heat and direct sun.
Inactive ingredients
water • ethylhexyl palmitate • neopentyl glycol diethylhexanoate • cyclopentasiloxane • talc • caprylic/capric triglyceride • HDI/trimethylol hexyllactone crosspolymer • propylene glycol • glycerin • PEG-30 dipolyhydroxystearate • butylene glycol • phenoxyethanol • fragrance • methylparaben • disteardimonium hectorite • polyglyceryl-3 beeswax • disodium stearoyl glutamate • propylparaben • lecithin • tocopherol • ascorbyl palmitate • glyceryl stearate • alcohol • juglans regia (walnut) seed extract • silica • laminaria digitata extract • glyceryl oleate • sodium hyaluronate • aluminum hydroxide • citric acid • sodium metabisulfite • BHT • glycyrrhiza glabra (licorice) root extract • ethylparaben • denatonium benzoate (may contain) ultramarines • iron oxides • titanium dioxide • mica
Questions or comments?
Call 1-800-550-0005 M – F 10am –8pm, S – S 12pm – 6pm EST.
PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - 20 Clair
VITALUMIÈRE
MOISTURE-RICH RADIANCE SUNSCREEN FLUID MAKEUP
BROAD SPECTRUM SPF 15
20 CLAIR
CHANEL
30 mL 1 FL. OZ.
PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - 30 Cendré
VITALUMIÈRE
MOISTURE-RICH RADIANCE SUNSCREEN FLUID MAKEUP
BROAD SPECTRUM SPF 15
30 CENDRÉ
CHANEL
30 mL 1 FL. OZ.
PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - 40 Beige
VITALUMIÈRE
MOISTURE-RICH RADIANCE SUNSCREEN FLUID MAKEUP
BROAD SPECTRUM SPF 15
40 BEIGE
CHANEL
30 mL 1 FL. OZ.
PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - 50 Naturel
VITALUMIÈRE
MOISTURE-RICH RADIANCE SUNSCREEN FLUID MAKEUP
BROAD SPECTRUM SPF 15
50 NATUREL
CHANEL
30 mL 1 FL. OZ.
PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - 07 Ivoire
VITALUMIÈRE
MOISTURE-RICH RADIANCE SUNSCREEN FLUID MAKEUP
BROAD SPECTRUM SPF 15
07 IVOIRE
CHANEL
30 mL 1 FL. OZ.
PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - 35 Soft Bisque
VITALUMIÈRE
MOISTURE-RICH RADIANCE SUNSCREEN FLUID MAKEUP
BROAD SPECTRUM SPF 15
35 SOFT BISQUE
CHANEL
30 mL 1 FL. OZ.
PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - 41 Natural Beige
VITALUMIÈRE
MOISTURE-RICH RADIANCE SUNSCREEN FLUID MAKEUP
BROAD SPECTRUM SPF 15
41 NATURAL BEIGE
CHANEL
30 mL 1 FL. OZ.
PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - 51 Tawny Beige
VITALUMIÈRE
MOISTURE-RICH RADIANCE SUNSCREEN FLUID MAKEUP
BROAD SPECTRUM SPF 15
51 TAWNY BEIGE
CHANEL
30 mL 1 FL. OZ.