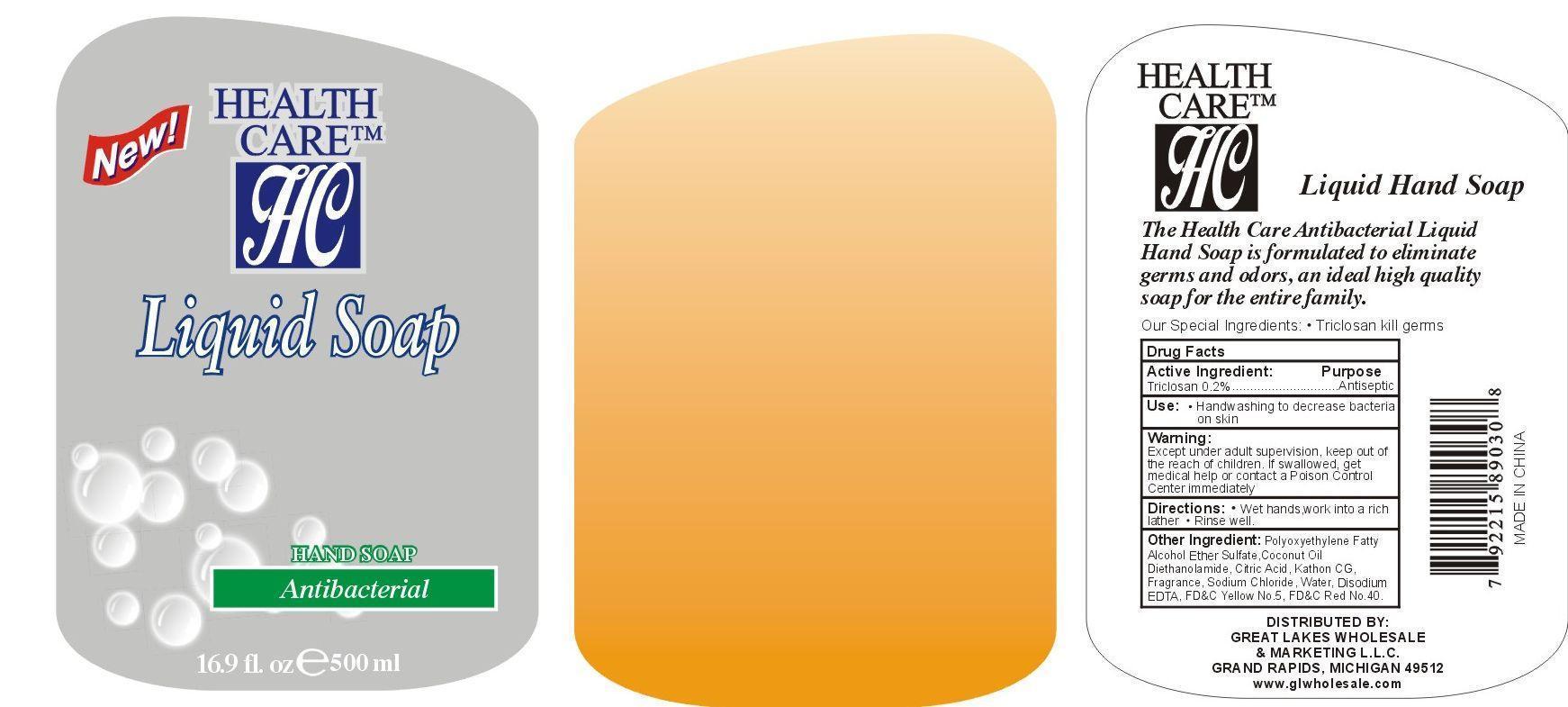
Label: LIQUID HAND CLEANSE- triclosan gel
- NDC Code(s): 57337-016-01
- Packager: Rejoice International, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated February 28, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- KEEP OUT OF REACH OF CHILDREN
- INDICATIONS & USAGE
- WARNINGS
- DOSAGE & ADMINISTRATION
- INACTIVE INGREDIENT
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
LIQUID HAND CLEANSE
triclosan gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:57337-016 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TRICLOSAN (UNII: 4NM5039Y5X) (TRICLOSAN - UNII:4NM5039Y5X) TRICLOSAN 10 mg in 500 mg Inactive Ingredients Ingredient Name Strength CETETH-7 (UNII: 4ST6952Q67) ETHYL SULFATE (UNII: P3LK0HF3B7) COCONUT OIL (UNII: Q9L0O73W7L) DIETHANOLAMINE (UNII: AZE05TDV2V) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) OCTHILINONE (UNII: 4LFS24GD0V) WATER (UNII: 059QF0KO0R) SODIUM CHLORIDE (UNII: 451W47IQ8X) EDETATE DISODIUM (UNII: 7FLD91C86K) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) FD&C RED NO. 40 (UNII: WZB9127XOA) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:57337-016-01 500 mg in 1 BOTTLE; Type 0: Not a Combination Product 07/05/2013 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M003 07/05/2013 Labeler - Rejoice International, Inc. (078741245) Establishment Name Address ID/FEI Business Operations China Ningbo Shangge Cosmetic Technology Corp. 529287434 manufacture(57337-016)