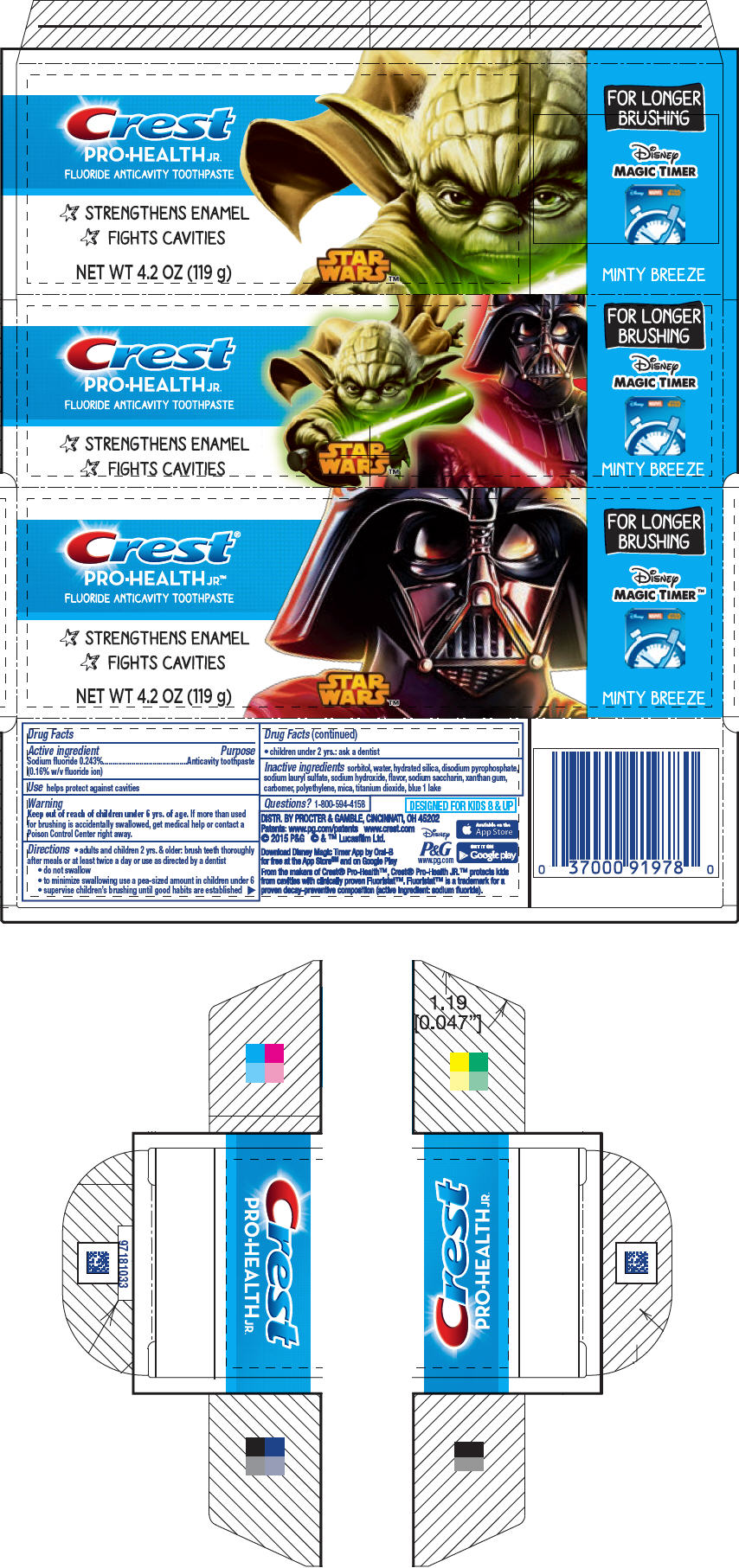
CREST PRO-HEALTH JR- sodium fluoride paste, dentifrice
Procter & Gamble Manufacturing Company
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Crest ® Pro-Health Jr.™
Directions
- adults and children 2 yrs. & older: brush teeth thoroughly after meals or at least twice a day or use as directed by a dentist
- do not swallow
- to minimize swallowing use a pea-sized amount in children under 6
- supervise children's brushing until good habits are established
- children under 2 yrs.: ask a dentist
| CREST PRO-HEALTH JR
sodium fluoride paste, dentifrice |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Procter & Gamble Manufacturing Company (004238200) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Procter & Gamble Manufacturing Company (Browns Summit) | 017745779 | manufacture(37000-870) | |
Revised: 10/2016
Document Id: 3ec4df18-dfd8-450d-e054-00144ff8d46c
Set id: 2353fd04-b0ac-4f23-a991-62cd34290388
Version: 2
Effective Time: 20161013
Procter & Gamble Manufacturing Company