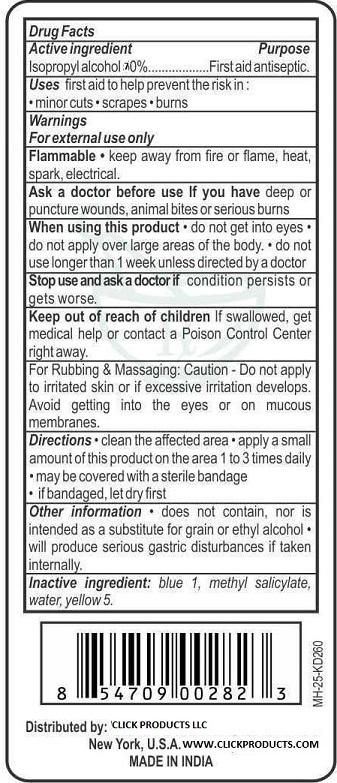
Label: RUBBING ALCOHOL 70 PERCENT WITH WINTERGREEN- isopropyl alcohol liquid
- NDC Code(s): 71611-304-12, 71611-304-16
- Packager: MY Sales LLC dba Click Products LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated April 18, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Purpose
- Uses
- Keep Out of Reach of Children
- WARNINGS
- ASK DOCTOR
- WHEN USING
- STOP USE
- Directions
- OTHER INFORMATION
- Inactive Ingredient
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
RUBBING ALCOHOL 70 PERCENT WITH WINTERGREEN
isopropyl alcohol liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:71611-304 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ISOPROPYL ALCOHOL (UNII: ND2M416302) (ISOPROPYL ALCOHOL - UNII:ND2M416302) ISOPROPYL ALCOHOL 70 mL in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) METHYL SALICYLATE (UNII: LAV5U5022Y) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71611-304-16 475 mL in 1 BOTTLE; Type 0: Not a Combination Product 02/22/2018 2 NDC:71611-304-12 355 mL in 1 BOTTLE; Type 0: Not a Combination Product 02/22/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M003 02/22/2018 Labeler - MY Sales LLC dba Click Products LLC (080766174)