CLOROX HEALTHCARE NASAL ANTISEPTIC SWABS- povidone iodine swabstick solution
Aplicare Products, LLC.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
0972 Clorox Healthcare Nasal Antiseptic Swabs
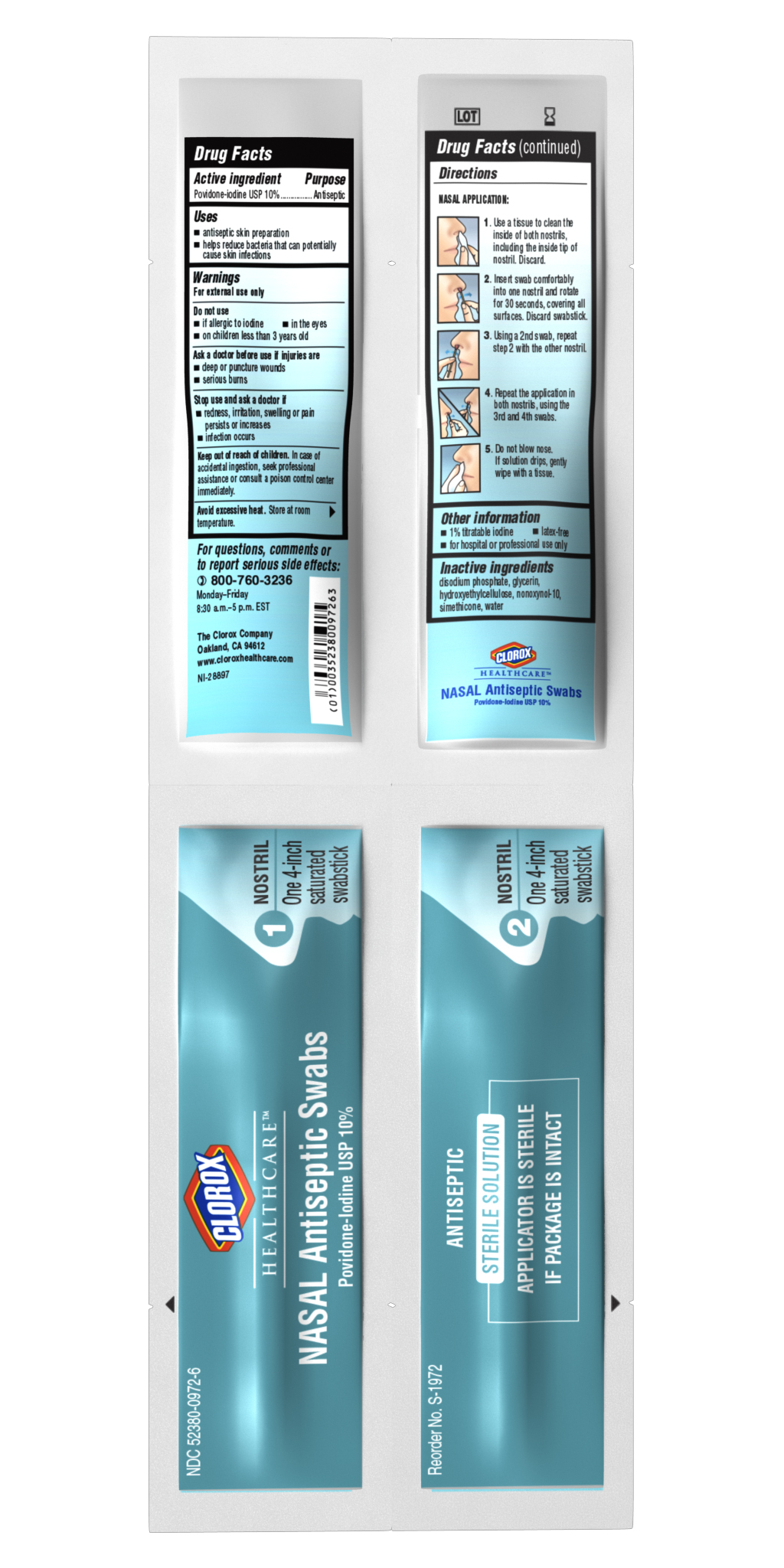
For external use only
Do not use
• if allergic to iodine
• in the eyes
• on children less than 3 years old
A sk a doctor before use if injuries are
• deep or puncture wounds
• serious burns
Stop use and ask doctor if
- redness, irritation, swelling or pain persists or increases
- infection occurs
Keep out of reach of children. In case of accidental ingestion, seek professional assistance or consult a poison control center immediately.
Avoid excessive heat. Store at room temperature.
Directions
NASAL APPLICATION:
1. Use a tissue to clean the inside of both nostrils including the inside tip of nostril. Discard.
2. Insert swab comfortably into one nostril and rotate for 30 seconds covering all surfaces. Discard swabstick.
3. Using a second swab, repeat step 2 with the other nostril.
4. Repeat the application in both nostrils using the 3rd and 4th swabs.
5. Do not blow nose. If solution drips gently wipe with a tissue.
Inactive ingredients
• disodium phosphate
• glycerin
• hydroxyethylcellulose
• nonoxynol-10
• simethicone
• water
| CLOROX HEALTHCARE NASAL ANTISEPTIC SWABS
povidone iodine swabstick solution |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Aplicare Products, LLC. (081054904) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Aplicare Products, LLC. | 081054904 | manufacture(52380-0972) | |
 CLOROX HEALTHCARE
CLOROX HEALTHCARE