Label: UNDA 220- grindelia, pilocarpus, salvia officinalis, senega officinalis, stramonium, valeriana officinalis liquid
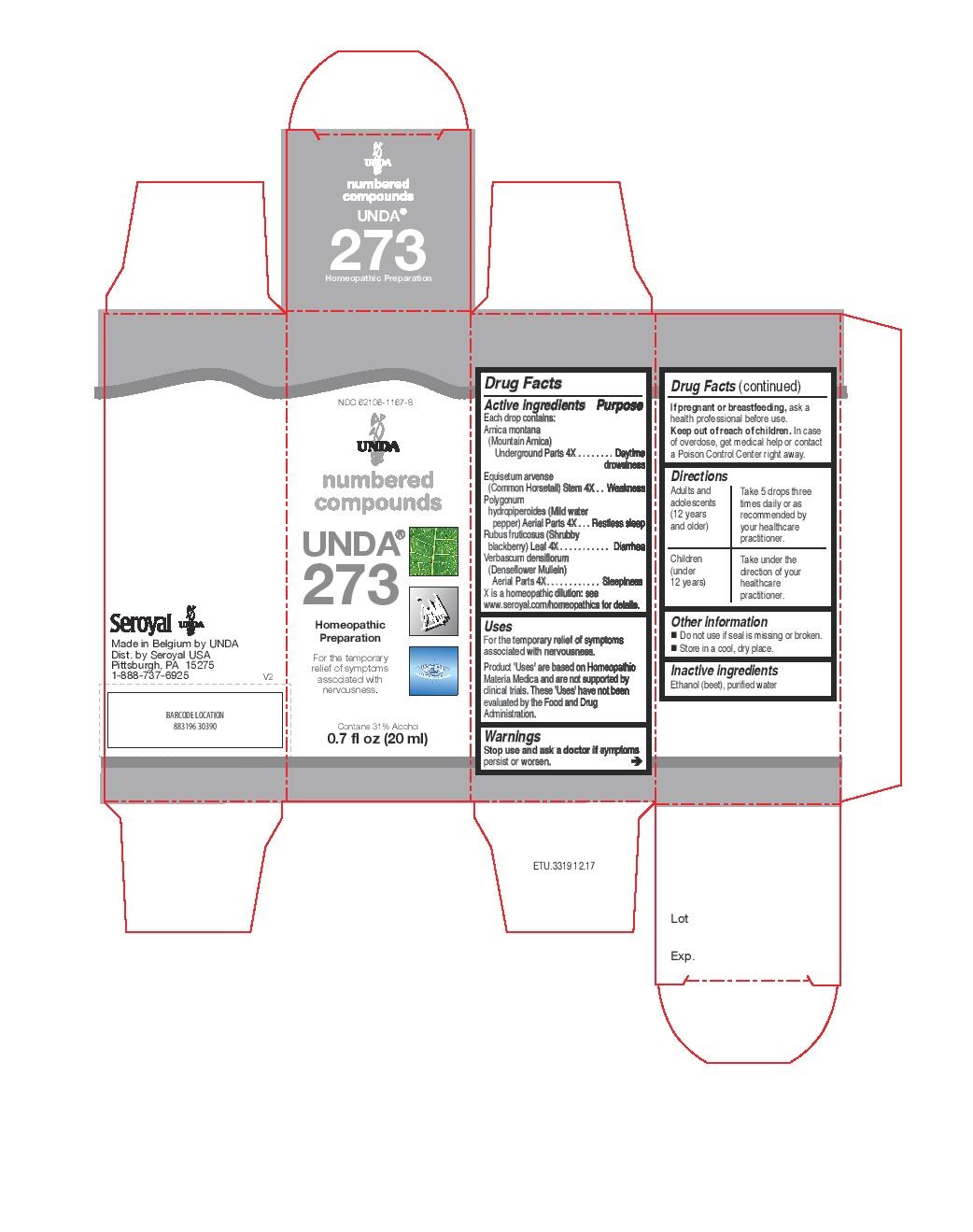
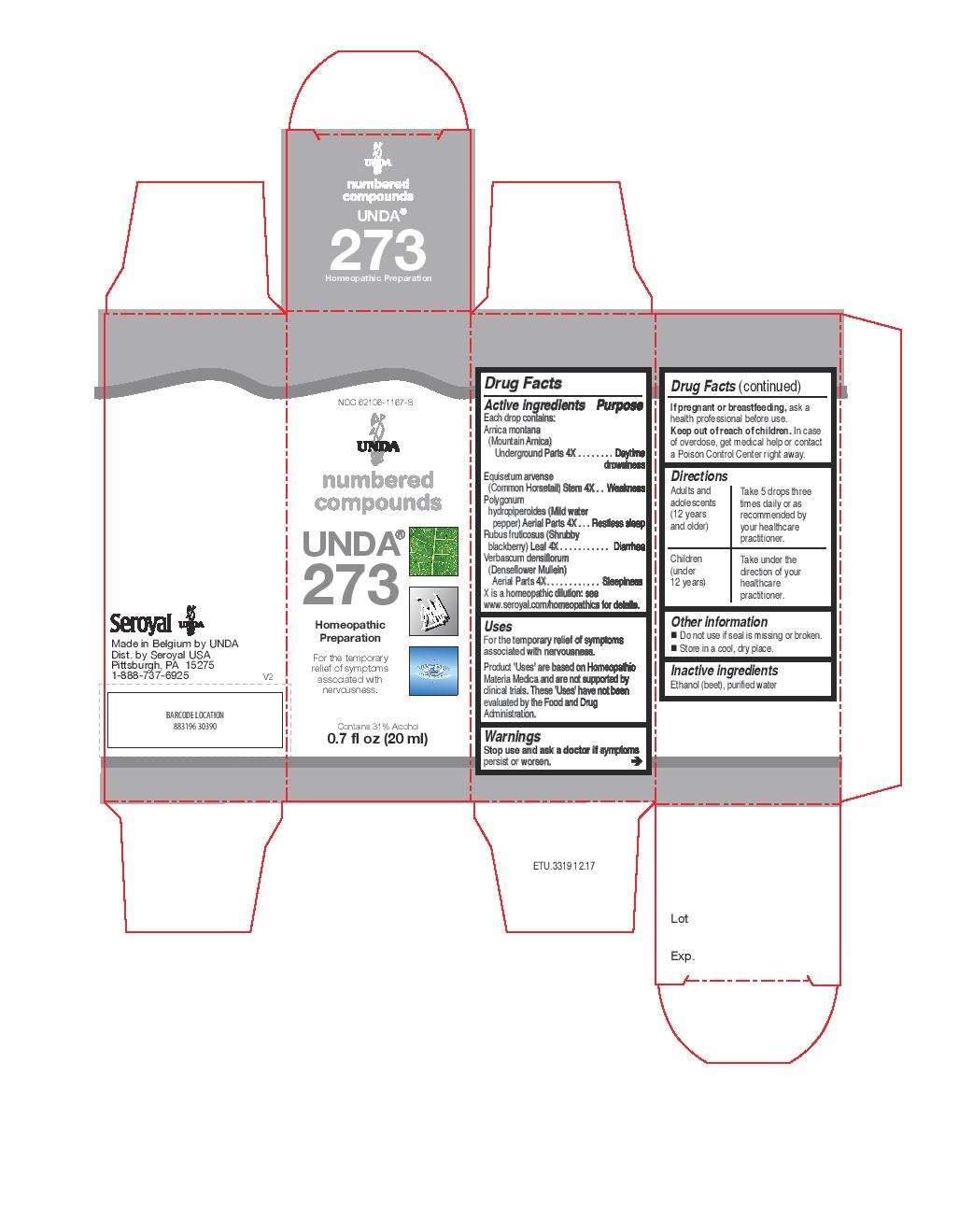
UNDA 273- arnica montana, equisetum arvense, polygonum hydropiperoides, rubus fruticosus, verbascum densiflorum liquid
- NDC Code(s): 62106-1157-8, 62106-1167-8
- Packager: Seroyal USA
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated November 9, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
-
WARNINGS
Warnings
Ask a doctor before use if you havePersistent or chronic cough such as occurs with smoking, asthma, chronic bronchitis, or emphysema.
Cough that is accompanied by excessive phlegm (mucus) or fever.
Stop use and ask a doctor if
Cough persists for more than 1 week, tends to recur, or is accompanied by a fever, rash, or
persistent headache. A persistent cough may be a sign of a serious condition.
Symptoms persist or worsen.If pregnant or breastfeeding, ask a health professional before use.
Keep out of reach of children. In case of overdose, get medical help or contact
a Poison Control Center right away. - STOP USE
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
- OVERDOSAGE
- INACTIVE INGREDIENT
- OTHER SAFETY INFORMATION
- DOSAGE & ADMINISTRATION
- INDICATIONS & USAGE
- ACTIVE INGREDIENT
- PURPOSE
- WARNINGS
- STOP USE
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
- OVERDOSAGE
- INACTIVE INGREDIENT
- OTHER SAFETY INFORMATION
- DOSAGE & ADMINISTRATION
- INDICATIONS & USAGE
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
UNDA 220
grindelia, pilocarpus, salvia officinalis, senega officinalis, stramonium, valeriana officinalis liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:62106-1157 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GRINDELIA HIRSUTULA FLOWERING TOP (UNII: IDB0NAZ6AI) (GRINDELIA HIRSUTULA FLOWERING TOP - UNII:IDB0NAZ6AI) GRINDELIA HIRSUTULA FLOWERING TOP 6 [hp_X] in 20 mL PILOCARPUS MICROPHYLLUS LEAF (UNII: TY68V0X4KL) (PILOCARPUS MICROPHYLLUS LEAF - UNII:TY68V0X4KL) PILOCARPUS MICROPHYLLUS LEAF 6 [hp_X] in 20 mL SAGE (UNII: 065C5D077J) (SAGE - UNII:065C5D077J) SAGE 6 [hp_X] in 20 mL DATURA STRAMONIUM (UNII: G6W4F0V8Z3) (DATURA STRAMONIUM - UNII:G6W4F0V8Z3) DATURA STRAMONIUM 6 [hp_X] in 20 mL VALERIAN (UNII: JWF5YAW3QW) (VALERIAN - UNII:JWF5YAW3QW) VALERIAN 6 [hp_X] in 20 mL POLYGALA SENEGA ROOT (UNII: M7T6H7D4IF) (POLYGALA SENEGA ROOT - UNII:M7T6H7D4IF) POLYGALA SENEGA ROOT 6 [hp_X] in 20 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALCOHOL (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62106-1157-8 1 in 1 CARTON 09/22/2015 1 20 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 09/22/2015 UNDA 273
arnica montana, equisetum arvense, polygonum hydropiperoides, rubus fruticosus, verbascum densiflorum liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:62106-1167 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ARNICA MONTANA ROOT (UNII: MUE8Y11327) (ARNICA MONTANA ROOT - UNII:MUE8Y11327) ARNICA MONTANA ROOT 4 [hp_X] in 20 mL EQUISETUM ARVENSE BRANCH (UNII: 1L0VKZ185E) (EQUISETUM ARVENSE BRANCH - UNII:1L0VKZ185E) EQUISETUM ARVENSE BRANCH 4 [hp_X] in 20 mL PERSICARIA PUNCTATA (UNII: D32E7AXD4R) (PERSICARIA PUNCTATA - UNII:D32E7AXD4R) PERSICARIA PUNCTATA 4 [hp_X] in 20 mL RUBUS FRUTICOSUS LEAF (UNII: YQ2S06L8S9) (RUBUS FRUTICOSUS LEAF - UNII:YQ2S06L8S9) RUBUS FRUTICOSUS LEAF 4 [hp_X] in 20 mL VERBASCUM DENSIFLORUM FLOWERING TOP (UNII: 8LR978PU7Y) (VERBASCUM DENSIFLORUM FLOWERING TOP - UNII:8LR978PU7Y) VERBASCUM DENSIFLORUM FLOWERING TOP 4 [hp_X] in 20 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62106-1167-8 1 in 1 CARTON 09/22/2015 1 20 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 09/22/2015 Labeler - Seroyal USA (018361118) Establishment Name Address ID/FEI Business Operations SAN’UP 401010287 manufacture(62106-1157, 62106-1167)