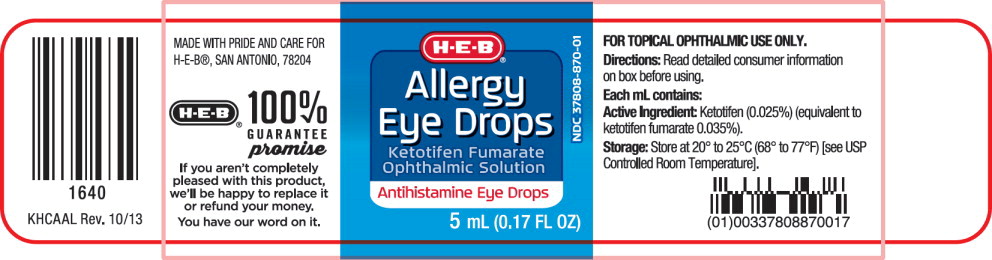
Label: ALLERGY EYE DROPS- ketotifen fumarate solution/ drops
-
Contains inactivated NDC Code(s)
NDC Code(s): 37808-870-01 - Packager: H E B
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated January 25, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Uses
-
Warnings
Do not use
- if solution changes color or becomes cloudy
- if you are sensitive to any ingredient in this product
- to treat contact lens related irritation
When using this product
- do not touch tip of container to any surface to avoid contamination
- remove contact lenses before use
- wait at least 10 minutes before reinserting contact lenses after use
- replace cap after each use
- Directions
- Other information
- Inactive ingredients
- Questions or comments?
- PRINCIPAL DISPLAY PANEL
-
PRINCIPAL DISPLAY PANEL
Principal Display Panel Text for Carton Label:
NDC 37808-870-01 Compare to
Claritin® Eye
active
ingredient*
H ■ E ■ B Logo®
Allergy
Eye Drops
Ketotifen Fumarate
Ophthalmic Solution
Antihistamine Eye Drops
Allergy Eye
Itch Relief
• Works in
Minutes
• Original
Prescription
Strength
• For ages
3 years
& Older
UP TO
12
HOURS
ITCH RELIEF
STERILE 5 mL (0.17 FL OZ)
-
INGREDIENTS AND APPEARANCE
ALLERGY EYE DROPS
ketotifen fumarate solution/ dropsProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:37808-870 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Ketotifen Fumarate (UNII: HBD503WORO) (Ketotifen - UNII:X49220T18G) Ketotifen 0.35 mg in 1 mL Inactive Ingredients Ingredient Name Strength Benzalkonium Chloride (UNII: F5UM2KM3W7) Glycerin (UNII: PDC6A3C0OX) Water (UNII: 059QF0KO0R) Hydrochloric Acid (UNII: QTT17582CB) Sodium Hydroxide (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:37808-870-01 1 in 1 CARTON 02/25/2014 1 5 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA077958 02/25/2014 Labeler - H E B (007924756) Registrant - Akorn Operating Company LLC (117693100) Establishment Name Address ID/FEI Business Operations Akorn 117696840 MANUFACTURE(37808-870) , ANALYSIS(37808-870) , STERILIZE(37808-870) , PACK(37808-870) , LABEL(37808-870)