Label: SUPRA SULFA III- sulfamethazine tablet, extended release
- NDC Code(s): 46066-123-02, 46066-123-03
- Packager: Aspen Veterinary Resources
- Category: PRESCRIPTION ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Animal Drug Application
Drug Label Information
Updated October 11, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
Supra Sulfa® III
(sulfamethazine)
SUSTAINED RELEASE BOLUS (72 HOURS)
Antibacterial
CAUTION: Federal law restricts this drug to use by or on the order of a licensed veterinarian.NOT FOR HUMAN USE
KEEP OUT OF REACH OF CHILDREN
Approved by FDA under NADA # 120-615Net Contents: 50 Boluses
EACH BOLUS CONTAINS: Sulfamethazine (formulated in a sustained release base), 495 grains (32.1 grams) -
INDICATIONS & USAGE
Supra Sulfa® III Boluses (sulfamethazine) are intended for oral administration to beef cattle and non-lactating dairy cattle (See RESIDUE WARNING Statement). Supra Sulfa® III Boluses are indicated for the treatment of the following diseases when caused by one or more of the following pathogenic organisms sensitive to sulfamethazine: Bacterial Pneumonia and Bovine Respiratory Disease Complex (Shipping Fever Complex) (Pasteurella spp.), Colibacillosis (Bacterial Scours) (E. Coli), Necrotic pododermatitis (Foot Rot), Calf Diphtheria (Fusobacterium necrophorum), Acute Metritis (Streptococcus spp.).
-
RESIDUE WARNING
RESIDUE WARNING: Treated animals intended for human consumption should not be slaughtered for food for at least 12 days after the last dose. Exceeding two (2) consecutive doses may cause violative tissue residues to remain beyond the withdrawal time. Do not use in female dairy cattle 20 months of age or older. Use of sulfamethazine in this class of cattle may cause milk residues. Do not use in calves under one (1) month of age or calves being fed an all-milk diet. Use in these classes of calves may cause violative residues to remain beyond the withdrawal time.
-
PRECAUTIONS
CAUTION: This drug, like all sulfonamides, may cause toxic reactions and irreparable injury unless administered with adequate and continuous supervision; follow recommended dosages carefully.
Fluid intake must be adequate at all times throughout the three-day therapy provided by the sustained release bolus. This product has not been shown to be effective for non-ruminating calves.
-
DOSAGE & ADMINISTRATION
DOSAGE AND ADMINISTRATION: Supra Sulfa® III Boluses (sulfamethazine) are designed to be administered orally to beef cattle and non-lactating dairy cattle (See RESIDUE WARNING Statement). Supra Sulfa® III Boluses should be given according to the following dosage schedule:
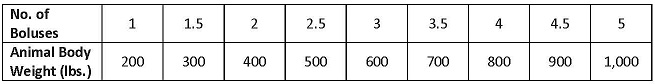
This bolus may be divided for better approximation of correct dose; however, care should be taken not to crush the bolus. Care should also be taken to ensure the entire dose has been swallowed by the animal. Observe animals following administration to ensure boluses are not regurgitated. Lubricate bolus before dosing animals.
Supra Sulfa® III Boluses are designed to provide a therapeutic sulfamethazine level in approximately 6 hours and persist in providing this level for 72 hours (3 days). After 72 hours, all animals should be re-examined for persistence of observable disease signs. If signs are present, consult a veterinarian. It is strongly recommended that a second dose be given to provide for an additional 72 hours of therapy particularly in those more severe cases. The dosage schedule should be used at each 72 hour interval.
- STORAGE AND HANDLING
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
SUPRA SULFA III
sulfamethazine tablet, extended releaseProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC:46066-123 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SULFAMETHAZINE (UNII: 48U51W007F) (SULFAMETHAZINE - UNII:48U51W007F) SULFAMETHAZINE 32.1 g Product Characteristics Color white Score 2 pieces Shape OVAL Size 83mm Flavor Imprint Code NA Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:46066-123-02 50 in 1 BOX 2 NDC:46066-123-03 100 in 1 BOX Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA120615 03/12/2012 Labeler - Aspen Veterinary Resources (627265361) Registrant - Bimeda Inc. (060492923) Establishment Name Address ID/FEI Business Operations Bimeda Inc. 060492923 manufacture


