TERRAMYCIN SCOURS- oxytetracycline hydrochloride tablet
Pfizer Animal Health
----------
Terramycin®
(oxytetracycline HCl)
Scours Tablets
Each tablet contains 250 mg of oxytetracycline HCl.
A broad-spectrum antibiotic for use in beef and dairy calves
Read Entire Package Outsert Carefully Before Using This Product
DESCRIPTION
Terramycin Scours Tablets is an oral formulation containing oxytetracycline, a versatile, broad-spectrum antibiotic that possesses potent antimicrobial activity, for use in beef and dairy calves.
INDICATIONS AND USAGE
Terramycin Scours Tablets are recommended for oral administration for the control and treatment of the following diseases in beef and dairy calves caused by organisms sensitive to oxytetracycline: bacterial enteritis caused by Salmonella typhimurium and Escherichia coli (colibacillosis) and bacterial pneumonia (shipping fever complex, pasteurellosis) caused by Pasteurella multocida.
WARNINGS
Discontinue treatment at least 7 days prior to slaughter. Not for use in lactating dairy cattle. A withdrawal period has not been established for this product in preruminating calves. Do not use in calves to be processed for veal.
PRECAUTIONS
Exceeding the recommended dosage level of 2 tablets per 100 lb of body weight every 12 hours (10 mg/lb of body weight daily), or administering at this recommended level for more than 4 consecutive days, may result in antibiotic residues beyond the withdrawal time.
Organisms may vary in their degree of susceptibility to any chemotherapy. If no improvement is observed after recommended treatment, diagnosis and susceptibility should be reexamined.
Rarely do side reactions or allergic manifestations occur in calves treated with Terramycin. If any unusual reactions are noted, discontinue use of the drug immediately and call a veterinarian.
Since bacteriostatic drugs may interfere with the bacterial action of penicillin, it is advisable to avoid giving Terramycin in conjunction with penicillin.
DOSAGE AND ADMINISTRATION
For control of bacterial enteritis and bacterial pneumonia orally administer 1 tablet per 100 lb of body weight every 12 hours (5 mg/ lb of body weight daily in divided doses) for up to 4 consecutive days.
For treatment of bacterial enteritis and bacterial pneumonia orally administer 2 tablets per 100 lb of body weight every 12 hours (10 mg/lb of body weight daily in divided doses) for up to 4 consecutive days.
Dosage should continue until the animal returns to normal and for 24–48 hours after symptoms have subsided. Treatment should not exceed 4 consecutive days.
Care of Sick Animals
The use of antibiotics in the management of disease is based on an accurate diagnosis and an adequate course of treatment. When properly used in the treatment of diseases caused by oxytetracycline-susceptible organisms, most animals treated with Terramycin Scours Tablets show a noticeable improvement within 24–48 hours. If improvement does not occur within this period of time, the diagnosis and course of treatment should be reevaluated. It is recommended that the diagnosis and treatment of animal diseases be carried out by a veterinarian. Since many diseases look alike but require different types of treatment, the use of professional veterinary and laboratory services can reduce treatment time, costs, and needless losses. Good housing, sanitation, and nutrition are important in the maintenance of healthy animals and are essential in the treatment of disease.
For Animal Use Only
Restricted Drug (California)—Use Only as Directed
Not For Human Use

NADA #11-060, Approved by FDA
Made in India
Distributed by:
Pfizer Animal Health
Div. of Pfizer Inc, NY, NY 10017
75-7948-03IN.00
June 2008
PRINCIPAL DISPLAY PANEL - 24 Tablet Bottle Label
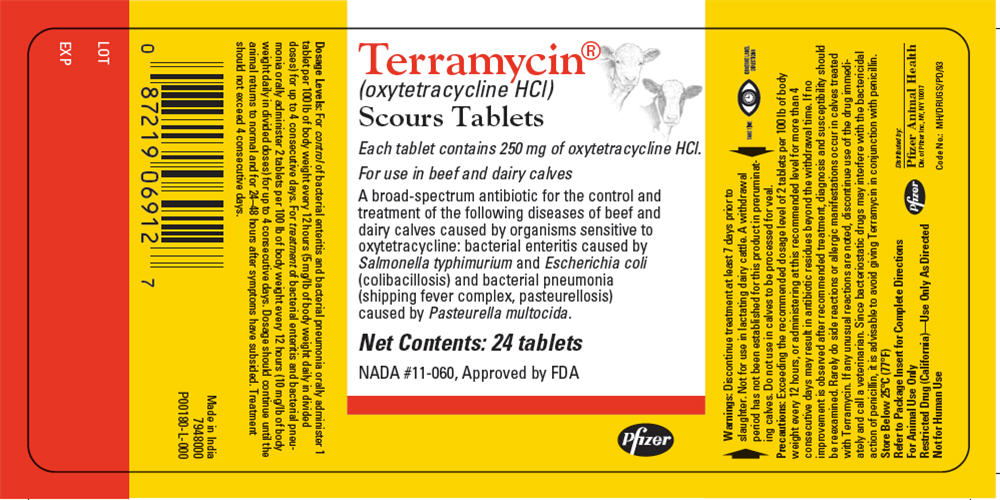
Terramycin®
(oxytetracycline HCl)
Scours Tablets
Each tablet contains 250 mg of oxytetracycline HCl.
For use in beef and dairy calves
A broad-spectrum antibiotic for the control and
treatment of the following diseases of beef and
dairy calves caused by organisms sensitive to
oxytetracycline: bacterial enteritis caused by
Salmonella typhimurium and Escherichia coli
(colibacillosis) and bacterial pneumonia
(shipping fever complex, pasteurellosis)
caused by Pasteurella multocida.
Net Contents: 24 tablets
NADA #11-060, Approved by FDA
Pfizer
| TERRAMYCIN
SCOURS
oxytetracycline hydrochloride tablet |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Pfizer Animal Health (039055157) |