ZMAX- azithromycin dihydrate powder, for suspension
Pfizer Laboratories Div Pfizer Inc
----------
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use ZMAX safely and effectively. See full prescribing information for ZMAX.
ZMAX® (azithromycin extended-release) for oral suspension Initial U.S. Approval: 1991 RECENT MAJOR CHANGES
INDICATIONS AND USAGEZmax is a macrolide antimicrobial drug indicated for mild to moderate infections caused by designated, susceptible bacteria:
Limitations of Use Azithromycin should not be used in patients with pneumonia who are judged to be inappropriate for oral therapy because of moderate to severe illness or risk factors (1.2). To reduce the development of drug-resistant bacteria and maintain the effectiveness of Zmax and other antibacterial drugs, Zmax should be used only to treat infections that are proven or strongly suspected to be caused by susceptible bacteria. (1.3) DOSAGE AND ADMINISTRATIONDOSAGE FORMS AND STRENGTHSBottle containing 2 g azithromycin for constitution with 60 mL of water (final concentration 27 mg/mL). (3) CONTRAINDICATIONSWARNINGS AND PRECAUTIONS
ADVERSE REACTIONSMost common adverse reactions (incidence ≥1%) are diarrhea/loose stools, nausea, abdominal pain, headache, and vomiting. (6.1, 6.2) To report SUSPECTED ADVERSE REACTIONS, contact Pfizer, Inc. at 1-800-438-1985 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch DRUG INTERACTIONSUSE IN SPECIFIC POPULATIONSSee 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling. Revised: 11/2021 |
FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
1.1 Acute Bacterial Sinusitis in Adults and Community-Acquired Pneumonia
Zmax (azithromycin) is a macrolide antibacterial drug indicated for the treatment of patients with mild to moderate infections caused by susceptible strains of the designated microorganisms in the specific conditions listed below. [See Clinical Studies (14)]
Acute bacterial sinusitis in adults due to Haemophilus influenzae, Moraxella catarrhalis or Streptococcus pneumoniae.
Community-acquired pneumonia in adults and pediatric patients six months of age or older due to Chlamydophila pneumoniae, Haemophilus influenzae, Mycoplasma pneumoniae or Streptococcus pneumoniae, in patients appropriate for oral therapy. Pediatric use in this indication is based on extrapolation of adult efficacy. [See Use in Specific Populations (8.4)]
1.2 Limitations of Use
Zmax is not recommended for use in patients with pneumonia who are judged to be inappropriate for oral therapy because of moderate to severe illness or risk factors such as any of the following:
- •
- patients with cystic fibrosis,
- •
- patients with nosocomial infections,
- •
- patients with known or suspected bacteremia,
- •
- patients requiring hospitalization,
- •
- elderly or debilitated patients, or
- •
- patients with significant underlying health problems that may compromise their ability to respond to their illness (including immunodeficiency or functional asplenia).
1.3 Usage
To reduce the development of drug-resistant bacteria and maintain the effectiveness of Zmax (azithromycin) and other antibacterial drugs, Zmax (azithromycin) should be used only to treat infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
2 DOSAGE AND ADMINISTRATION
2.1 Adults
Zmax should be taken as a single 2 g dose. Zmax provides a full course of antibacterial therapy in a single oral dose. It is recommended that Zmax be taken on an empty stomach (at least 1 hr before or 2 hr following a meal).
2.2 Pediatric Patients
For pediatric patients 6 months and older, Zmax should be taken as a single dose of 60 mg/kg body weight for patients weighing less than 34 kg. It is recommended that Zmax be taken on an empty stomach (at least 1 hr before or 2 hrs following a meal).
Pediatric patients weighing 34 kg should receive the adult dose (2 g).
| Dosing Calculated on 60 mg/kg as a single dose for Children <34 kg* | ||
|---|---|---|
| Weight | 27 mg/mL Dose | |
| Kg | Dose | Volume |
| (mg) | (mL) | |
|
||
|
5 |
270 |
10 |
|
7 |
405 |
15 |
|
9 |
540 |
20 |
|
11 |
675 |
25 |
|
14 |
810 |
30 |
|
16 |
945 |
35 |
|
18 |
1080 |
40 |
|
20 |
1215 |
45 |
|
23 |
1350 |
50 |
|
25 |
1485 |
55 |
|
27 |
1620 |
60 |
|
30 |
1755 |
65 |
|
32 |
1890 |
70 |
|
≥34 |
2000 |
Consume entire contents of bottle |
2.3 Additional Treatment after Vomiting with Zmax
In the event that a patient vomits within 5 minutes of administration, the health care provider should consider additional antibiotic treatment since there would be minimal absorption of azithromycin. Since insufficient data exist on absorption of azithromycin if a patient vomits between 5 and 60 minutes following administration, alternative therapy should be considered. Neither a second dose of Zmax nor alternative treatment is warranted if vomiting occurs ≥60 minutes following administration, in patients with normal gastric emptying. In patients with delayed gastric emptying, alternative therapy should be considered.
2.4 Instructions for the Pharmacist
Constitute with 60 mL of water and replace cap. Shake bottle well before dispensing. Do not refrigerate. Constituted suspension should be consumed within 12 hr.
For pediatric dosing in patients weighing less than 34 kg, use of a dosing device is recommended. The pharmacist should inform the patient's caregiver that any suspension remaining after dosing MUST be discarded.
3 DOSAGE FORMS AND STRENGTHS
Each bottle of Zmax contains azithromycin dihydrate equivalent to 2 g of azithromycin. After constitution with 60 mL of water, each mL of suspension contains 27 mg of azithromycin. The suspension is a white or off-white color and has a cherry/banana flavor.
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity
Serious allergic reactions, including angioedema, anaphylaxis, Acute Generalized Exanthematous Pustulosis (AGEP), Stevens Johnson syndrome, and toxic epidermal necrolysis have been reported in patients on azithromycin therapy using other formulations. Fatalities have been reported. Cases of Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS) have also been reported. Despite initially successful symptomatic treatment of the allergic symptoms, when symptomatic therapy was discontinued, the allergic symptoms recurred soon thereafter in some patients without further azithromycin exposure. These patients required prolonged periods of observation and symptomatic treatment. The relationship of these episodes to the long tissue half-life of azithromycin and subsequent exposure to antigen has not been determined.
If an allergic reaction occurs, appropriate therapy should be instituted. Physicians should be aware that reappearance of the allergic symptoms may occur when symptomatic therapy is discontinued.
5.2 Hepatotoxicity
Abnormal liver function, hepatitis, cholestatic jaundice, hepatic necrosis, and hepatic failure have been reported, some of which have resulted in death. Discontinue azithromycin immediately if signs and symptoms of hepatitis occur.
5.3 Infantile Hypertrophic Pyloric Stenosis (IHPS)
Following the use of azithromycin in neonates (treatment up to 42 days of life), IHPS has been reported. Direct parents and caregivers to contact their physician if vomiting or irritability with feeding occurs.
5.4 QT Prolongation
Prolonged cardiac repolarization and QT interval, imparting a risk of developing cardiac arrhythmia and torsades de pointes, have been seen in treatment with macrolides, including azithromycin. Cases of torsades de pointes have been spontaneously reported during postmarketing surveillance in patients receiving azithromycin. Providers should consider the risk of QT prolongation which can be fatal when weighing the risks and benefits of azithromycin for at-risk groups including:
- •
- patients with known prolongation of the QT interval, a history of torsades de pointes, congenital long QT syndrome, bradyarrhythmias or uncompensated heart failure
- •
- patients on drugs known to prolong the QT interval
- •
- patients with ongoing proarrhythmic conditions such as uncorrected hypokalemia or hypomagnesemia, clinically significant bradycardia, and in patients receiving Class IA (quinidine, procainamide) or Class III (dofetilide, amiodarone, sotalol) antiarrhythmic agents
Elderly patients may be more susceptible to drug-associated effects on the QT interval.
5.5 Cardiovascular Death
Some observational studies have shown an approximately two-fold increased short-term potential risk of acute cardiovascular death in adults exposed to azithromycin relative to other antibacterial drugs, including amoxicillin. The five-day cardiovascular mortality observed in these studies ranged from 20 to 400 per million azithromycin treatment courses. This potential risk was noted to be greater during the first five days of azithromycin use and does not appear to be limited to those patients with preexisting cardiovascular diseases. The data in these observational studies are insufficient to establish or exclude a causal relationship between acute cardiovascular death and azithromycin use. Consider balancing this potential risk with treatment benefits when prescribing Zmax.
5.6 Clostridioides difficile-Associated Diarrhea (CDAD)
Clostridioides difficile-associated diarrhea (CDAD) has been reported with use of nearly all antibacterial agents, including Zmax, and may range in severity from mild diarrhea to fatal colitis. Treatment with antibacterial agents alters the normal flora of the colon leading to overgrowth of C. difficile.
C. difficile produces toxins A and B which contribute to the development of CDAD. Hypertoxin producing strains of C. difficile cause increased morbidity and mortality, as these infections can be refractory to antimicrobial therapy and may require colectomy. CDAD must be considered in all patients who present with diarrhea following antibiotic use. Careful medical history is necessary since CDAD has been reported to occur over two months after the administration of antibacterial agents.
If CDAD is suspected or confirmed, ongoing antibiotic use not directed against C. difficile may need to be discontinued. Appropriate fluid and electrolyte management, protein supplementation, antibiotic treatment of C. difficile, and surgical evaluation should be instituted as clinically indicated.
5.7 Exacerbation of Myasthenia Gravis
Exacerbation of symptoms of myasthenia gravis and new onset of myasthenic syndrome have been reported in patients receiving azithromycin therapy.
5.8 Gastrointestinal Disturbances
A higher incidence of gastrointestinal adverse events (8 of 19 subjects) was observed when Zmax was administered to a limited number of subjects with GFR<10 mL/min. [See Use in Specific Populations (8.6)]
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in labeling:
- •
- Hypersensitivity [see Warnings and Precautions (5.1)]
- •
- Hepatotoxicity [see Warnings and Precautions (5.2)]
- •
- Infantile Hypertrophic Pyloric Stenosis (IHPS) [see Warnings and Precautions (5.3)]
- •
- QT Prolongation [see Warnings and Precautions (5.4)]
- •
- Cardiovascular Death [see Warnings and Precautions (5.5)]
- •
- Clostridioides difficile-Associated Diarrhea (CDAD) [see Warnings and Precautions (5.6)]
- •
- Exacerbation of Myasthenia Gravis [see Warnings and Precautions (5.7)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Adults:
The data described below reflect exposure to Zmax in 728 adult patients. All patients received a single 2 g oral dose of Zmax. The population studied had community-acquired pneumonia and acute bacterial sinusitis.
In controlled clinical trials with Zmax, the majority of the reported treatment-related adverse reactions were gastrointestinal in nature and mild to moderate in severity.
Overall, the most common treatment-related adverse reactions in adult patients receiving a single 2 g dose of Zmax were diarrhea/loose stools (12%), nausea (4%), abdominal pain (3%), headache (1%), and vomiting (1%). The incidence of treatment-related gastrointestinal adverse reactions was 17% for Zmax and 10% for pooled comparators.
Treatment-related adverse reactions following Zmax treatment that occurred with a frequency of <1% included the following:
Cardiovascular: Palpitations, chest pain
Gastrointestinal: Constipation, dyspepsia, flatulence, gastritis, oral moniliasis
Genitourinary: Vaginitis
Nervous system: Dizziness, vertigo
General: Asthenia
Allergic: Rash, pruritus, urticaria
Special senses: Taste perversion
Pediatric Patients:
The data described below reflect exposure to Zmax in 907 pediatric patients. The population was 3 months to 12 years of age. All patients received a single 60 mg/kg oral dose of Zmax.
As in adults, the most common treatment-related adverse reactions in pediatric subjects were gastrointestinal in nature. The pediatric subjects all received a single 60 mg/kg dose of Zmax.
In a trial with 450 pediatric subjects (ages 3 months to 48 months), vomiting (11%), diarrhea (10%) loose stools (9%), and abdominal pain (2%) were the most frequently reported treatment-related gastrointestinal adverse reactions. Many treatment related gastrointestinal adverse reactions with an incidence greater than 1% began on the day of dosing in these subjects [43% (68/160)] and most [53% (84/160)] resolved within 48 hr of onset. Treatment-related adverse events that were not gastrointestinal, occurring with a frequency ≥1% were: rash (5%), anorexia (2%), fever (2%), and dermatitis (2%).
In a second trial of 337 pediatric subjects, ages 2 years to 12 years, the most frequently reported treatment-related adverse reactions also included vomiting (14%), diarrhea (7%), loose stools (2%), nausea (4%) and abdominal pain (4%).
A third trial investigated the tolerability of two different concentrations of azithromycin oral suspension in 120 pediatric subjects (ages 3 months to 48 months), all of whom were treated with azithromycin. The study evaluated the hypothesis that a more dilute, less viscous formulation (the recommended 27 mg/mL concentration of Zmax) is less likely to induce vomiting in young children than a more concentrated suspension used in other pediatric studies. The vomiting rate for subjects taking the dilute concentration azithromycin was 3% (2/61). The rate was numerically lower but not statistically different from the vomiting for the more concentrated suspension. Across both treatment arms, the only treatment-related adverse events with a frequency of ≥1% were vomiting (6%, 7/120) and diarrhea (2%, 2/120).
Treatment-related adverse reactions with a frequency of <1% following Zmax treatment in all 907 pediatric subjects in the Phase 3 studies were:
Body as a whole: Chills, fever, flu syndrome, headache;
Digestive: Abnormal stools, constipation, dyspepsia, flatulence, gastritis, gastrointestinal disorder, hepatitis;
Hematologic and lymphatic: Leukopenia;
Nervous system: Agitation, emotional liability, hostility, hyperkinesia, insomnia, irritability, paresthesia, Somnolence;
Respiratory: Asthma, bronchitis, cough, dyspnea, pharyngitis, rhinitis;
Skin and appendages: Dermatitis, fungal dermatitis, maculopapular rash, pruritus, urticaria;
Special senses: Otitis media, taste perversion;
Urogenital: Dysuria.
6.2 Postmarketing Experience with Other Azithromycin Products
The following adverse reactions have been identified during post-approval use of azithromycin. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Adverse reactions reported with azithromycin during the postmarketing period in adult and/or pediatric patients for which a causal relationship may not be established include:
Allergic: Arthralgia, edema, urticaria and angioedema.
Cardiovascular: Palpitations and arrhythmias including ventricular tachycardia and hypotension. There have been reports of QT prolongation, torsades de pointes, and cardiovascular death.
Gastrointestinal: Anorexia, constipation, dyspepsia, flatulence, vomiting/diarrhea, pseudomembranous colitis, pancreatitis, oral candidiasis, pyloric stenosis, and rare reports of tongue discoloration.
General: Asthenia, paresthesia, fatigue, malaise and anaphylaxis.
Genitourinary: Interstitial nephritis, acute renal failure and vaginitis.
Hematopoietic: Thrombocytopenia, mild neutropenia.
Liver/biliary: Adverse reactions related to hepatic dysfunction have been reported in postmarketing experience with azithromycin. [See Warnings and Precautions (5.2)].
Nervous system: Convulsions, dizziness/vertigo, headache, somnolence, hyperactivity, nervousness, agitation and syncope.
Psychiatric: Aggressive reaction and anxiety.
Skin/appendages: Pruritus, rash, photosensitivity, serious skin reactions including erythema multiforme, AGEP, Stevens-Johnson syndrome, toxic epidermal necrolysis, and DRESS.
Special senses: Hearing disturbances including hearing loss, deafness and/or tinnitus and reports of taste/smell perversion and/or loss.
6.3 Laboratory Abnormalities
In subjects with normal baseline values, the following clinically significant laboratory abnormalities (irrespective of drug relationship) were reported in Zmax clinical trials in adults and pediatric patients:
Adults:
Laboratory abnormalities with an incidence of greater than or equal to 1%: reduced lymphocytes and increased eosinophils; reduced bicarbonate. Laboratory abnormalities with an incidence of less than 1%: leukopenia, neutropenia, elevated bilirubin, AST, ALT, BUN, creatinine, alterations in potassium. Where follow-up was provided, changes in laboratory tests appeared to be reversible.
Pediatric Patients:
Laboratory abnormalities with an incidence of greater than or equal to 1%: elevated eosinophils, BUN, and potassium; decreased lymphocytes; and alterations in neutrophils; with an incidence of less than 1%: elevated SGOT, SGPT and creatinine; decreased potassium; and alterations in sodium and glucose.
7 DRUG INTERACTIONS
7.1 Nelfinavir
Co-administration of nelfinavir at steady-state with a single oral dose of azithromycin resulted in increased azithromycin serum concentrations. Although a dose adjustment of azithromycin is not recommended when administered in combination with nelfinavir, close monitoring for known adverse reactions of azithromycin, such as liver enzyme abnormalities and hearing impairment, is warranted. [see Adverse Reactions (6)]
7.2 Warfarin
Spontaneous postmarketing reports suggest that concomitant administration of azithromycin may potentiate the effects of oral anticoagulants such as warfarin, although the prothrombin time was not affected in the dedicated drug interaction study with azithromycin and warfarin. Prothrombin times should be carefully monitored while patients are receiving azithromycin and oral anticoagulants concomitantly.
7.3 Potential Drug-Drug Interaction with Macrolides
Interactions with digoxin, colchicine or phenytoin have not been reported in clinical trials with azithromycin. No specific drug interaction studies have been performed to evaluate potential drug-drug interaction. However, drug interactions have been observed with other macrolide products. Until further data are developed regarding drug interactions when digoxin, colchicine or phenytoin are used with azithromycin careful monitoring of patients is advised.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Available data from published literature and postmarketing experience over several decades with azithromycin use in pregnant women have not identified any drug-associated risks for major birth defects, miscarriage, or adverse maternal or fetal outcomes (see Data). Developmental toxicity studies with azithromycin in rats, mice, and rabbits showed no drug-induced fetal malformations at doses up to 1, 0.5, and 0.4 times, respectively, an adult human oral dose of 2 g based on body surface area. Decreased viability and delayed development were observed in the offspring of pregnant rats administered azithromycin from day 6 of pregnancy through weaning at a dose equivalent to an adult human oral dose of 2 g based on body surface area (see Data).
The estimated background risk of major birth defects and miscarriage for the indicated populations is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Data
Human Data
Available data from published observational studies, case series, and case reports over several decades do not suggest an increased risk for major birth defects, miscarriage, or adverse maternal or fetal outcomes with azithromycin use in pregnant women. Limitations of these data include the lack of randomization and inability to control for confounders such as underlying maternal disease and maternal use of concomitant medications.
Animal Data
Azithromycin administered during the period of organogenesis did not cause fetal malformations in rats and mice at oral doses up to 200 mg/kg/day (moderately maternally toxic). Based on body surface area, this dose is approximately equivalent to one or one-half of, respectively, the single adult human oral dose of 2 g. In rabbits administered azithromycin at oral doses of 10, 20, and 40 mg/kg/day during organogenesis, reduced maternal body weight and food consumption were observed in all groups; no evidence of fetotoxicity or teratogenicity was observed at these doses, the highest of which is estimated to be 0.4 times an adult human oral dose of 2 g based on body surface area.
In a pre- and postnatal development study, azithromycin was administered orally to pregnant rats from day 6 of pregnancy until weaning at doses of 50 or 200 mg/kg/day. Maternal toxicity (reduced food consumption and body weight gain; increased stress at parturition) was observed at the higher dose. Effects in the offspring were noted at 200 mg/kg/day during the postnatal development period (decreased viability, delayed developmental landmarks). These effects were not observed in a pre- and postnatal rat study when up to 200 mg/kg/day of azithromycin was given orally beginning on day 15 of pregnancy until weaning.
8.2 Lactation
Risk Summary
Azithromycin is present in human milk (see Data). Non-serious adverse reactions have been reported in breastfed infants after maternal administration of azithromycin (see Clinical Considerations). There are no available data on the effects of azithromycin on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for Zmax and any potential adverse effects on the breastfed infant from Zmax or from the underlying maternal condition.
Clinical Considerations
Advise women to monitor the breastfed infant for diarrhea, vomiting, or rash.
Data
Azithromycin breastmilk concentrations were measured in 20 women after receiving a single 2 g oral dose of azithromycin during labor. Breastmilk samples collected on days 3 and 6 postpartum as well as 2 and 4 weeks postpartum revealed the presence of azithromycin in breastmilk up to 4 weeks after dosing. In another study, a single dose of azithromycin 500 mg was administered intravenously to 8 women prior to incision for cesarean section. Breastmilk (colostrum) samples obtained between 12 and 48 hours after dosing revealed that azithromycin persisted in breastmilk up to 48 hours.
8.4 Pediatric Use
Safety and effectiveness in the treatment of pediatric patients under 6 months of age have not been established.
Community-Acquired Pneumonia: The safety and effectiveness of Zmax have been established in pediatric patients 6 months of age or older with community-acquired pneumonia due to Chlamydophila pneumoniae, Mycoplasma pneumoniae, Haemophilus influenzae or Streptococcus pneumoniae. Use of Zmax for these patients is supported by evidence from adequate and well-controlled studies of Zmax in adults with additional safety and pharmacokinetic data in pediatric patients. [See Dosage and Administration (2.2), Adverse reactions (6), Clinical Pharmacology (12.3)]
8.5 Geriatric Use
Data collected from the azithromycin capsule and tablet formulations indicate that a dosage adjustment does not appear to be necessary for older patients with normal renal function (for their age) and hepatic function receiving treatment with Zmax.
In clinical trials of Zmax, 17% of subjects were at least 65 years of age (214/1292) and 5% of subjects (59/1292) were at least 75 years of age. No overall differences in safety or effectiveness were observed between these subjects and younger subjects. Elderly patients may be more susceptible to development of torsades de pointes arrhythmia than younger patients. [See Warnings and Precautions (5.4)]
8.6 Renal Impairment
No dosage adjustment is recommended for patients GFR >10 mL/min. Caution should be exercised when Zmax is administered to patients with GFR <10 mL/min, due to a higher incidence of gastrointestinal adverse events (8 of 19 subjects) observed in a limited number of subjects with GFR <10 mL/min. [See Clinical Pharmacology (12)]
8.7 Gender
The impact of gender on the pharmacokinetics of azithromycin has not been evaluated for Zmax. However, previous studies have demonstrated no significant differences in the disposition of azithromycin between male and female subjects. No dosage adjustment of Zmax is recommended based on gender.
10 OVERDOSAGE
Adverse reactions experienced at higher than recommended doses were similar to those seen at normal doses. In the event of overdosage, general symptomatic and supportive measures are indicated as required.
11 DESCRIPTION
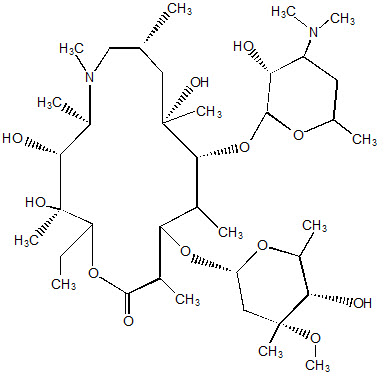
Zmax (azithromycin extended-release) for oral suspension contains the active ingredient azithromycin (as azithromycin dihydrate), an azalide, a subclass of macrolide antibacterial drug. Azithromycin has the chemical name (2R,3S,4R,5R,8R,10R,11R,12S,13S,14R)13-[(2,6-Dideoxy-3-C-methyl-3-O-methyl-α-L-ribo-hexopyranosyl) oxy]-2-ethyl-3,4,10-trihydroxy-3,5,6,8,10,12,14-heptamethyl-11-[[3,4,6-trideoxy-3-(dimethylamino)-β-D-xylo-hexopyranosyl]oxy]-1-oxa-6-azacyclopentadecan-15-one. Azithromycin is derived from erythromycin; however, it differs chemically from erythromycin in that a methyl-substituted nitrogen atom is incorporated into the lactone ring. Its molecular formula is C38H72N2O12, and its molecular weight is 749.0. Azithromycin has the following structural formula:
Azithromycin, as the dihydrate, is a white crystalline powder with a molecular formula of C38H72N2O12∙2H2O and a molecular weight of 785.0.
Zmax is a single-dose, extended-release formulation of microspheres for oral suspension containing azithromycin (as azithromycin dihydrate) and the following excipients: glyceryl behenate, poloxamer 407, sucrose, sodium phosphate tribasic anhydrous, magnesium hydroxide, hydroxypropyl cellulose, xanthan gum, colloidal silicon dioxide, titanium dioxide, artificial cherry flavor, and artificial banana flavor.
Note: Each bottle of Zmax 2 g for oral suspension contains approximately 148 mg of sodium and 19 g of sucrose. Constituted Zmax oral suspension contains approximately 2 mg/mL of sodium and 0.26 g/mL of sucrose.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Azithromycin is a macrolide antibacterial drug. [See Clinical Pharmacology (12.4)]
12.2 Pharmacodynamics
Cardiac Electrophysiology
QTc interval prolongation was studied in a randomized, placebo-controlled parallel trial in 116 healthy subjects who received either chloroquine (1000 mg) alone or in combination with azithromycin (500 mg, 1000 mg, and 1500 mg once daily). Co-administration of azithromycin increased the QTc interval in a dose- and concentration-dependent manner. In comparison to chloroquine alone, the maximum mean (95% upper confidence bound) increases in QTcF were 5 (10) ms, 7 (12) ms and 9 (14) ms with the co-administration of 500 mg, 1000 mg and 1500 mg azithromycin, respectively.
12.3 Pharmacokinetics
Zmax is an extended-release microsphere formulation. Based on data obtained from studies evaluating the pharmacokinetics of azithromycin in healthy adult subjects a higher peak serum concentration (Cmax) and greater systemic exposure (AUC 0–24) of azithromycin are achieved on the day of dosing following a single 2 g dose of Zmax versus 1.5 g of azithromycin tablets administered over 3 days (500 mg/day) or 5 days (500 mg on day 1, 250 mg/day on days 2–5) [Table 2]. Consequently, due to these different pharmacokinetic profiles, Zmax is not interchangeable with azithromycin tablet 3-day and 5-day dosing regimens.
| Pharmacokinetic Parameter* | Azithromycin Regimen | ||
|---|---|---|---|
| Zmax
[N=41]† | 3-day ‡
[N=12] | 5-day ‡
[N=12] |
|
| ¶ Total AUC for the 1-day, 3-day and 5-day regimens | |||
| SD = standard deviation Cmax = maximum serum concentration Tmax = time to Cmax AUC = area under concentration vs. time curve t1/2 = terminal serum half-life |
|||
|
Cmax (mcg/mL) |
0.821 |
0.441 |
0.434 |
|
Tmax§ (hr) |
5.0 |
2.5 |
2.5 |
|
AUC0–24
|
8.62 |
2.58 |
2.60 |
|
AUC0–∞
|
20.0 |
17.4 |
14.9 |
|
t1/2 (hr) |
58.8 |
71.8 |
68.9 |
Absorption
The bioavailability of Zmax relative to azithromycin immediate release (IR) (powder for oral suspension) was 83%. On average, peak serum concentrations were achieved approximately 2.5 hr later following Zmax administration and were lower by 57%, compared to 2 g azithromycin IR. Thus, single 2 g doses of Zmax and azithromycin IR are not bioequivalent and are not interchangeable.
Effect of food on absorption: A high-fat meal increased the rate and extent of absorption of a 2 g dose of Zmax (115% increase in Cmax, and 23% increase in AUC0–72) compared to the fasted state. A standard meal also increased the rate of absorption (119% increase in Cmax) and with less effect on the extent of absorption (12% increase in AUC0–72) compared to administration of a 2 g Zmax dose in the fasted state.
Distribution
The serum protein binding of azithromycin is concentration dependent, decreasing from 51% at 0.02 mcg/mL to 7% at 2 mcg/mL. Following oral administration, azithromycin is widely distributed throughout the body with an apparent steady-state volume of distribution of 31.1 L/kg.
Azithromycin concentrates in fibroblasts, epithelial cells, macrophages, and circulating neutrophils and monocytes. Higher azithromycin concentrations in tissues than in plasma or serum have been observed. White blood cell and lung exposure data in humans following a single 2 g dose of Zmax in adults are shown in Table 3. Following a 2 g single dose of Zmax, azithromycin achieved higher exposure (AUC0–120) in mononuclear leukocytes (MNL) and polymorphonuclear leukocytes (PMNL) than in serum. The azithromycin exposure (AUC0–72) in lung tissue and alveolar cells (AC) was approximately 100 times that in serum; and the exposure in epithelial lining fluid (ELF) was also higher (approximately 2–3 times) than in serum. The clinical significance of this distribution data is unknown.
| A single 2 g dose of Zmax | ||||
|---|---|---|---|---|
| Abbreviation: WBC: white blood cells; MNL: mononuclear leukocytes; PMNL: polymorphonuclear leukocytes; ELF: Epithelial lining fluid | ||||
|
WBC |
Cmax (mcg/mL) |
AUC0–24 (mcg∙hr/mL) |
AUC0–120 (mcg∙hr/mL) |
Ct=120* (mcg/mL) |
|
MNL† |
116 (40.2) |
1790 (540) |
4710 (1100) |
16.2 (5.51) |
|
PMNL† |
146 (66.0) |
2080 (650) |
10000 (2690) |
81.7 (23.3) |
|
|
|
|
|
|
|
LUNG |
Cmax (mcg/mL) |
AUC0–24 (mcg∙hr/mL) |
AUC0–72 (mcg∙hr/mL) | |
|
ALVEOLAR CELL‡ |
669 |
7028 |
20403 |
- |
|
ELF‡ |
3.2 |
17.6 |
131 |
- |
|
|
|
|
|
|
|
Cmax (mcg/g) |
AUC0–24 (mcg∙hr/g) |
AUC0–72 (mcg∙hr/g) | ||
|
LUNG TISSUE‡ |
37.9 |
505 |
1693 |
- |
Following a regimen of 500 mg of azithromycin tablets on the first day and 250 mg daily for 4 days, only very low concentrations were noted in cerebrospinal fluid (less than 0.01 mcg/mL) in the presence of non-inflamed meninges.
Metabolism
In vitro and in vivo studies to assess the metabolism of azithromycin have not been performed.
Excretion
Serum azithromycin concentrations following a single 2 g dose of Zmax declined in a polyphasic pattern with a terminal elimination half-life of 59 hr. The prolonged terminal half-life is thought to be due to a large apparent volume of distribution.
Biliary excretion of azithromycin, predominantly as unchanged drug, is a major route of elimination. Over the course of a week, approximately 6% of the administered dose appears as unchanged drug in urine.
Specific Populations
Patients with Renal Impairment
Azithromycin pharmacokinetics were investigated in 42 adults (21 to 85 years of age) with varying degrees of renal impairment. Following the oral administration of a single 1.0 g dose of azithromycin (4 × 250 mg capsules), the mean Cmax and AUC0–120 were 5.1% and 4.2% higher, respectively in subjects with GFR 10 to 80 mL/min compared to subjects with normal renal function (GFR >80 mL/min). The mean Cmax and AUC0–120 were 61% and 35% higher, respectively in subjects with GFR <10 mL/min compared to subjects with normal renal function. [See Use in Specific Populations Renal Impairment (8.6)]
Patients with Hepatic Impairment
The pharmacokinetics of azithromycin in subjects with hepatic impairment has not been established.
Pediatric Patients
The pharmacokinetics of azithromycin were characterized following a single 60 mg/kg dose of Zmax in pediatric patients aged 3 months to 16 years. Although there was high inter-patient variability in systemic exposure (AUC and Cmax) across the age groups studied, individual azithromycin AUC and Cmax values in pediatric patients were comparable to or higher than those following administration of 2 g Zmax in adults (Table 4). [See Use in Specific Populations (8.4)]
| Treatment Group | Pharmacokinetic Parameters | |||
|---|---|---|---|---|
| Cmax
(mcg/mL) | Tmax*
(hr) | AUC(0–24)
(mcg∙hr/mL) | AUC(0–∞)
(mcg∙hr/mL) |
|
| Empty stomach = dosed with Zmax at least 1 hr before or 2 hr after a meal (Groups I–VI) | ||||
| Fed = dosed with Zmax within 5 minutes of consuming an age-appropriate high-fat breakfast (Group VII) | ||||
|
Group 1 (N = 6) |
0.74 (0.20) |
3 (3–3) |
6.29 (1.17) |
14.1 (2.16) |
|
Group 2† (N = 6) |
1.88† (0.50) |
3 (3–3) |
19.7† (5.35) |
37.3 (12.9) |
|
Group 3 (N = 6) |
1.23 (0.42) |
3 (3–6) |
12.9 (3.79) |
22.4 (5.96) |
|
Group 4 (N = 6) |
1.13 (0.34) |
3 (3–6) |
13.0 (4.21) |
22.2 (6.89) |
|
Group 5 (N = 6) |
1.65 (0.38) |
3 (3–6) |
16.0 (4.99) |
30.1 (10.7) |
|
Group 6 (N = 6) |
0.98 (0.35) |
3 (3–6) |
11.0 (4.78) |
21.3 (9.37) |
|
Pooled 1–6 (N = 36) |
1.27 (0.53) |
3 (3–6) |
13.1 (5.78) |
25.2 (10.7) |
|
Group 7‡ (N = 7) |
1.41 (0.62) |
3 (1.5–3.1) |
7.43 (3.00) |
18.9 (3.57) |
Drug Interaction Studies
A drug interaction study was performed with Zmax and antacids. All other drug interaction studies were performed with azithromycin immediate release (IR) formulations (capsules and tablets, doses ranging from 500 to 1200 mg) and other drugs likely to be co-administered. The effects of co-administration of azithromycin on the pharmacokinetics of other drugs are shown in Table 5 and the effects of other drugs on the pharmacokinetics of azithromycin are shown in Table 6.
When used at therapeutic doses, azithromycin IR had a minimal effect on the pharmacokinetics of atorvastatin, carbamazepine, cetirizine, didanosine, efavirenz, fluconazole, indinavir, midazolam, nelfinavir, sildenafil, theophylline (intravenous and oral), triazolam, trimethoprim/sulfamethoxazole or zidovudine (Table 5). Although the drug interaction studies were not conducted with Zmax, similar modest effect as observed with IR formulation are expected since the total exposure to azithromycin is comparable for Zmax and other azithromycin IR regimens. Therefore, no dosage adjustment of drugs listed in Table 5 is recommended when co-administered with Zmax.
Nelfinavir significantly increased the Cmax and AUC of azithromycin following co-administration with azithromycin IR 1200 mg (Table 6). However, no dose adjustment of azithromycin is recommended when Zmax is co-administered with nelfinavir.
Pharmacokinetic and/or pharmacodynamic interactions with the drugs listed below have not been reported in clinical trials with azithromycin; however, no specific drug interaction studies have been performed to evaluate potential drug-drug interaction. Nonetheless, pharmacokinetic and/or pharmacodynamic interactions with these drugs have been observed with other macrolide products. Until further data are developed, careful monitoring of patients is advised when azithromycin and these drugs are used concomitantly: digoxin, colchicine, ergotamine or dihydroergotamine, cyclosporine, hexobarbital and phenytoin.
| Co-administered Drug | Dose of Co-administered Drug | Dose of Azithromycin* | n | Ratio (with/without Azithromycin) of Co-administered Drug Pharmacokinetic Parameters (90% CI); No Effect = 1.00 | |
|---|---|---|---|---|---|
| Mean Cmax | Mean AUC | ||||
|
Atorvastatin |
10 mg/day for 8 days |
500 mg/day orally on days 6–8 |
12 |
0.83 |
1.01 |
|
Carbamazepine |
200 mg/day for 2 days, then 200 mg twice a day for 18 days |
500 mg/day orally for days 16–18 |
7 |
0.97 |
0.96 |
|
Cetirizine |
20 mg/day for 11 days |
500 mg orally on day 7, then 250 mg/day on days 8–11 |
14 |
1.03 |
1.02 |
|
Didanosine |
200 mg orally twice a day for 21 days |
1,200 mg/day orally on days 8–21 |
6 |
1.44 |
1.14 |
|
Efavirenz |
400 mg/day for 7 days |
600 mg orally on day 7 |
14 |
1.04† |
0.95† |
|
Fluconazole |
200 mg orally single dose |
1,200 mg orally single dose |
18 |
1.04 |
1.01 |
|
Indinavir |
800 mg three times a day for 5 days |
1,200 mg orally on day 5 |
18 |
0.96 |
0.90 |
|
Midazolam |
15 mg orally on day 3 |
500 mg/day orally for 3 days |
12 |
1.27 |
1.26 |
|
Nelfinavir |
750 mg three times a day for 11 days |
1,200 mg orally on day 9 |
14 |
0.90 |
0.85 |
|
Sildenafil |
100 mg on days 1 and 4 |
500 mg/day orally for 3 days |
12 |
1.16 |
0.92 |
|
Theophylline |
4 mg/kg IV on days 1, 11, 25 |
500 mg orally on day 7, then 250 mg/day on days 8–11 |
10 |
1.19 |
1.02 |
|
Theophylline |
300 mg orally twice a day for 15 days |
500 mg orally on day 6, then 250 mg/day on days 7–10 |
8 |
1.09 |
1.08 |
|
Triazolam |
0.125 mg on day 2 |
500 mg orally on day 1, then 250 mg/day on day 2 |
12 |
1.06† |
1.02† |
|
Trimethoprim/
|
160 mg/800 mg/day orally for 7 days |
1,200 mg orally on day 7 |
12 |
0.85 |
0.87 |
|
Zidovudine |
500 mg/day orally for 21 days |
600 mg/day oraly for 14 days |
5 |
1.12 |
0.94 |
|
Zidovudine |
500 mg/day orally for 21 days |
1,200 mg/day orally for 14 days |
4 |
1.31 |
1.30 |
| Co-administered Drug | Dose of Co-administered Drug | Dose of Azithromycin* | n | Ratio (with/without co-administered drug) of Azithromycin Pharmacokinetic Parameters (90% CI); No Effect = 1.00 | |
|---|---|---|---|---|---|
| Mean Cmax | Mean AUC | ||||
|
Efavirenz |
400 mg/day for 7 days |
600 mg orally on day 7 |
14 |
1.22 |
0.92† |
|
Fluconazole |
200 mg orally single dose |
1,200 mg orally single dose |
18 |
0.82 |
1.07 |
|
Nelfinavir |
750 mg three times a day 11 days |
1,200 mg orally on day 9 |
14 |
2.36 |
2.12 |
|
Aluminum and Magnesium hydroxide |
20 mL regular strength, single dose |
2 g Zmax, single dose |
39 |
0.99 |
0.99 |
12.4 Microbiology
Mechanism of Action
Azithromycin acts by binding to the 23S rRNA of the 50S ribosomal subunit of susceptible microorganisms inhibiting bacterial protein synthesis and impeding the assembly of the 50S ribosomal subunit.
Resistance
Azithromycin demonstrates cross resistance with erythromycin. The most frequently encountered mechanism of resistance to azithromycin is modification of the 23S rRNA target, most often by methylation. Ribosomal modifications can determine cross resistance to other macrolides, lincosamides and streptogramin B (MLSB phenotype).
Azithromycin has been shown to be active against the following microorganisms, both in vitro and in clinical infections. [See Indications and Usage (1)].
Gram-Positive Bacteria
Streptococcus pneumoniae
Gram-Negative Bacteria
Haemophilus influenzae
Moraxella catarrhalis
"Other" Bacteria
Chlamydophila pneumoniae
Mycoplasma pneumoniae
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term studies in animals have not been performed to evaluate carcinogenic potential. Azithromycin has shown no mutagenic potential in standard laboratory tests: mouse lymphoma assay, human lymphocyte clastogenic assay, and mouse bone marrow clastogenic assay. In fertility studies conducted in male and female rats, oral administration of azithromycin for 64 to 66 days (males) or 15 days (females) prior to and during cohabitation resulted in decreased pregnancy rate at 20 and 30 mg/kg/day when both males and females were treated with azithromycin. This minimal effect on pregnancy rate (approximately 12% reduction compared to concurrent controls) did not become more pronounced when the dose was increased from 20 to 30 mg/kg/day (approximately 0.1 to 0.2 times the adult human oral dose of 2 g based on body surface area) and it was not observed when only one animal in the mated pair was treated. There were no effects on any other reproductive parameters, and there were no effects on fertility at 10 mg/kg/day. The relevance of these findings to patients being treated with azithromycin at the doses and durations recommended in the prescribing information is uncertain.
13.2 Animal Toxicology and/or Pharmacology
Phospholipidosis (intracellular phospholipid accumulation) has been observed in some tissues of mice, rats, and dogs given multiple doses of azithromycin. It has been demonstrated in numerous organ systems (e.g., eye, dorsal root ganglia, liver, gallbladder, kidney, spleen, and/or pancreas) in dogs treated with azithromycin at doses which, expressed on the basis of body surface area, are approximately one-sixth the recommended adult human dose, and in rats treated at doses approximately one-fourth the recommended adult human dose. This effect has been shown to be reversible after cessation of azithromycin treatment. Based on the pharmacokinetic data, phospholipidosis has been seen in the rat (50 mg/kg/day dose) at the observed maximal plasma concentration of 1.3 mcg/mL (1.6 times the observed Cmax of 0.821 mcg/mL at the adult dose of 2 g.). Similarly, it has been shown in the dog (10 mg/kg/day dose) at the observed maximal serum concentration of 1 mcg/mL (1.2 times the observed Cmax of 0.821 mcg/mL at the adult dose of 2 g).
Phospholipidosis was also observed in neonatal rats dosed for 18 days at 30 mg/kg/day, which is less than the pediatric dose of 60 mg/kg based on the surface area. It was not observed in neonatal rats treated for 10 days at 40 mg/kg/day with mean maximal serum concentrations of 1.86 mcg/mL, approximately 1.5 times the Cmax of 1.27 mcg/mL at the pediatric dose. Phospholipidosis has been observed in neonatal dogs (10 mg/kg/day) at maximum mean whole blood concentrations of 3.54 mcg/mL, approximately 3 times the pediatric dose Cmax.
The significance of the finding for animals and for humans is unknown.
14 CLINICAL STUDIES
14.1 Acute Bacterial Maxillary Sinusitis
Adult subjects with a diagnosis of acute bacterial maxillary sinusitis were evaluated in a randomized, double-blind, multicenter study; a maxillary sinus tap was performed on all subjects at baseline. Clinical evaluations were conducted for all subjects at the TOC visit, 7 to 14 days post-treatment. Two hundred seventy (270) subjects were treated with a single 2 g oral dose of Zmax and 268 subjects were treated with levofloxacin, 500 mg orally once daily for 10 days. A subject was considered a cure if signs and symptoms related to the acute infection had resolved, or if clinical improvement was such that no additional antibiotics were deemed necessary. The clinical response for the primary population, Clinical Per Protocol Subjects, is presented below.
| ZMAX | LEVOFLOXACIN | |
|---|---|---|
| RESPONSE AT TOC | N = 255 | N = 254 |
|
CURE |
241 (94.5%) |
236 (92.9%) |
|
FAILURE |
14 (5.5%) |
18 (7.1%) |
Clinical response by pathogen in the Bacteriologic Per Protocol population is presented below.
| Zmax | Levofloxacin | |||
|---|---|---|---|---|
| Pathogen | N | Cure | N | Cure |
|
S. pneumoniae |
37 |
36 (97.3%) |
39 |
36 (92.3%) |
|
H. influenzae |
27 |
26 (96.3%) |
30 |
30 (100.0%) |
|
M. catarrhalis |
8 |
8 (100.0%) |
11 |
10 (90.9%) |
14.2 Community-Acquired Pneumonia
Adult subjects with a diagnosis of mild-to-moderate community-acquired pneumonia were evaluated in two, randomized, double-blind, multicenter studies. In both studies, clinical and microbiologic evaluations were conducted for all subjects at the Test of Cure (TOC) visit, 7 to 14 days post-treatment. In Trial 1, 247 subjects were treated with a single 2 g oral dose of Zmax and 252 subjects were treated with clarithromycin extended-release, 1 g orally once daily for 7 days. In Trial 2, 211 subjects were treated with a single 2.0 g oral dose of Zmax and 212 subjects were treated with levofloxacin, 500 mg orally once daily for 7 days. A patient was considered a cure if signs and symptoms related to the acute infection had resolved, or if clinical improvement was such that no additional antibiotics were deemed necessary; in addition, the chest x-ray performed at the TOC visit was to be either improved or stable. The clinical response at TOC for the primary population, Clinical Per Protocol Subjects, is presented in the table below.
| Zmax | Comparator | |
|---|---|---|
|
Zmax vs. Clarithromycin extended-release |
N=202 |
N=209 |
|
Cure |
187 (92.6%) |
198 (94.7%) |
|
Failure |
15 (7.4%) |
11 (5.3%) |
|
Zmax vs. Levofloxacin |
N=174 |
N=189 |
|
Cure |
156 (89.7%) |
177 (93.7%) |
|
Failure |
18 (10.3%) |
12 (6.3%) |
Clinical response by pathogen in the Bacteriologic Per Protocol population, across both studies, is presented below:
| Pathogen | Zmax | Comparators | ||
|---|---|---|---|---|
| N | Cure | N | Cure | |
|
S. pneumoniae |
33 |
28 (84.8%) |
39 |
35 (89.7%) |
|
H. influenzae |
30 |
28 (93.3%) |
34 |
31 (91.2%) |
|
C. pneumoniae |
40 |
37 (92.5%) |
53 |
50 (94.3%) |
|
M. pneumoniae |
33 |
30 (90.9%) |
39 |
38 (97.4%) |
16 HOW SUPPLIED/STORAGE AND HANDLING
NDC 0069-4170-34 for combined adult and pediatric use is supplied in bottles containing 2 g of azithromycin and should be constituted with 60 mL of water.
Storage
Before constitution, store dry powder at or below 30°C (86°F).
After constitution, store suspension at 25°C (77°F); excursions permitted to 15–30°C (59–86°F) [see USP Controlled Room Temperature]. Do not refrigerate or freeze.
Constituted suspension should be consumed within 12 hr. For adult patients, the entire bottle should be consumed. For pediatric patients, any suspension remaining after dosing MUST be discarded.
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
General Patient Counseling
- •
- Patients should be instructed to take Zmax on an empty stomach (at least 1 hr before or 2 hr following a meal).
- •
- To ensure accurate dosing for children, use of a dosing spoon, medicine syringe, or cup is recommended.
- •
- Patients should be told that Zmax needs time to work, so the patient may not feel better right away. If the patient's symptoms do not improve in a few days, the patient or their guardian should call their healthcare provider.
- •
- Patients should be instructed to immediately contact a physician if any signs of an allergic reaction occur.
- •
- Direct parents or caregivers to contact their physician if vomiting and irritability with feeding occurs in the infant.
- •
- Diarrhea is a common problem caused by antibiotics which usually ends when the antibiotic is discontinued. Sometimes after starting treatment with antibiotics, patients can develop watery and bloody stools (with or without stomach cramps and fever) even as late as two or more months after having taken the last dose of the antibiotic. If this occurs, patients should contact their physician as soon as possible.
- •
- Patients who vomit within the first hr should contact their health care provider about further treatment.
- •
- Keep bottle tightly closed. Store at room temperature. Use within 12 hr of constitution. Shake bottle well before use. Adult patients should consume the entire contents of the bottle; pediatric patients should take the recommended dose and MUST discard any unused portion.
- •
- Patients should be advised that Zmax may be taken without regard to antacids containing magnesium hydroxide and/or aluminum hydroxide.
Patients should be counseled that antibacterial drugs including Zmax should only be used to treat bacterial infections. They do not treat viral infections (e.g., the common cold). Not taking the complete prescribed dose may (1) decrease the effectiveness of the immediate treatment and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by Zmax or other antibacterial drugs in the future.
This product's label may have been updated. For current full prescribing information, please visit www.pfizer.com.
LAB-0384-15.0
Patient Information
Zmax® (azithromycin extended release)
Oral suspension
Read the Patient Information that comes with Zmax® carefully before you or your child take it. This leaflet does not take the place of talking with your healthcare provider about you or your child's medical condition or treatment. Only your healthcare provider can decide if Zmax is right for you or your child.
What is Zmax?
Zmax is an antibiotic that kills certain bacteria. Zmax is dosed differently from other antibiotics.
You take just one dose, one time.
- •
- Day 1: Take Zmax in one dose. Zmax starts working.
- •
- Days 2 – 3: As with most antibiotics, you may not feel better right away.
- •
- After Day 3: Zmax continues to work over time. If your symptoms are not better, call your healthcare provider.
Zmax is used in adults and in children over the age of 6 months against bacteria to treat certain kinds of pneumonia (lung infections)
Zmax is used in adults against bacteria to treat sinus infections.
Zmax only works against bacteria. It does not work against viruses, like the common cold or flu.
Zmax has not been studied in children under 6 months of age.
Who should not take Zmax?
- •
- You or your child should not take Zmax if allergic to:
- o
- anything in Zmax. See the end of this leaflet for a complete list of ingredients in Zmax.
- o
- antibiotics like erythromycin or telithromycin (Ketek®).
Talk with your healthcare provider or pharmacist if you have questions about your medicine allergies.
Before you start Zmax
Tell your healthcare provider about all your or your child's medical problems including if you or your child:
- •
- have liver problems.
- •
- have kidney problems.
- •
- have myasthenia gravis.
- •
- are pregnant, or might be pregnant. It is not known if Zmax could harm your baby.
- •
- are breast-feeding.
Contact your healthcare provider immediately if you are giving Zmax to a young child (less than 6 weeks of age) and he or she vomits or becomes irritable when fed.
Tell your healthcare provider about all the medicines you take, including prescription and non-prescription medicines, vitamins and herbal supplements.
Zmax and other medicines may affect each other causing side effects. Zmax may affect the way other medicines work, and other medicines may affect how Zmax works.
Especially tell your healthcare provider if you take:
- •
- nelfinavir
- •
- a blood thinner (warfarin)
- •
- digoxin
- •
- colchicine
- •
- phenytoin
- •
- an antacid that contains aluminum or magnesium
Know the medicines you take. Keep a list of your medicines and show it to your healthcare provider or pharmacist when you get a new prescription.
Do I need to prepare Zmax?
- •
- If you get Zmax in liquid form, it is ready to take.
- •
- If you get Zmax as dry powder, you must add water to the bottle
- •
-
before you take it. To prepare Zmax:
- 1.
- Open the bottle: To open the bottle, press down on the cap and twist.
- 2.
- Use a measuring cup to add 60 mL (1/4 cup) water to the Zmax bottle.
- 3.
- Tightly close the bottle and shake to mix it.
How do I take Zmax?
- •
- Keep Zmax at room temperature between 59°F to 86°F (15° to 30°C).
- •
- Shake the bottle well before using.
- •
- Take Zmax or give it to your child within 12 hr after it has been prepared by the pharmacy or you add water to the powder.
- •
- Take Zmax or give it to your child exactly how your healthcare provider prescribes it. This will help to treat you or your child's infection and decrease the chance that Zmax or other antibiotics will not work to treat infections in the future.
- •
- Adults: take all the medicine in the bottle.
- •
- Children: give your child the amount of Zmax prescribed by your healthcare provider and throw away the rest of the medicine.
- •
- To be sure that you give your child the right dose of Zmax, use a dosing spoon, medicine syringe, or cup.
- •
- Take Zmax on an empty stomach (at least 1 hr before eating or 2 hr after eating).
- •
- You can take antacids with Zmax.
- •
- If you or your child throws up (vomits) within one hr of taking Zmax, call your healthcare provider right away to see if more medicine is needed. Do not give your child more Zmax unless your healthcare provider tells you to.
- •
- If your child takes too much Zmax, call your healthcare provider right away or go to the nearest hospital emergency room.
How will I know Zmax is working?
Zmax needs time to work, so you or your child may not feel better right away. If you or your child's symptoms do not get better in a few days, call your healthcare provider.
What are the possible side effects of Zmax?
Zmax may cause serious side effects. These happened in a small number of patients. Call your healthcare provider right away or get emergency treatment if you or your child have any of the following:
- •
-
Serious allergic reaction or serious skin reaction: Get emergency help right away if you or your child has:
- o
- Skin rash (hives), sores in your mouth, or your skin blisters and peels
- o
- Trouble swallowing,
- o
- Swelling of your face, eyes, lips, tongue or throat
- o
- Wheezing or trouble breathing
- o
- New onset of fever and swollen lymph nodes
These symptoms could go away and then come back.
- •
- Diarrhea: Call your healthcare provider right away if you have diarrhea that does not go away, is severe, watery, or has blood in it. Diarrhea can occur as late as two or more months after you take an antibiotic such as Zmax.
- •
-
Serious heart rhythm changes that can be life-threatening, including heart stopping (cardiac arrest), QT prolongation, torsades de pointes, feeling that your heart is pounding or racing (palpitations), chest discomfort, or irregular heartbeat: Tell your healthcare provider right away if you or your child feel a fast or irregular heartbeat, get dizzy or faint. This has been seen with other antibiotics like Zmax.
Zmax may cause a rare heart problem known as prolongation of the QT interval. This condition can cause an abnormal heartbeat and can be very dangerous. The chances of this happening are higher in people:- o
- who are elderly
- o
- with a family history of prolonged QT interval
- o
- with low blood potassium
- o
- who take certain medicines to control heart rhythm (antiarrhythmics)
The most common side effects in adults are:
- •
- Diarrhea/loose stools
- •
- Nausea
- •
- Stomach pain
- •
- Headache
- •
- Vomiting
The most common side effects in children are:
- •
- Vomiting
- •
- Diarrhea/loose stools
- •
- Nausea
- •
- Stomach pain
Tell your healthcare provider if you have any side effects that bother your or your child, or that does not go away.
These are not all of the possible side effects with Zmax. For a list of all reported side effects, ask your healthcare provider or pharmacist.
General information about Zmax
Healthcare providers sometimes prescribe medicines for conditions that are not in the patient leaflets. Do not use Zmax for anything other than what your healthcare provider prescribed. Do not give it to other people, even if they have the same symptoms you have. It may harm them.
This Patient Information leaflet is a summary of the most important information about Zmax. For more information, talk with your healthcare provider. You can ask your healthcare provider or pharmacist for information about Zmax that is written for healthcare professionals. For more information, go to our website at www.zmaxinfo.com or call 1-800-438-1985.
What is in Zmax?
Active ingredient: azithromycin dihydrate
Inactive ingredients: glyceryl behenate, poloxamer 407, sucrose, sodium phosphate tribasic anhydrous, magnesium hydroxide, hydroxypropyl cellulose, xanthan gum, colloidal silicon dioxide, titanium dioxide, artificial cherry flavor, and artificial banana flavor
Brand names are registered trademarks of their respective owners.
Coumadin® is a registered trademark of Bristol-Myers Squibb, Inc.
Ketek® is a registered trademark of Aventis Pharmaceuticals Inc.
This product's label may have been updated. For current full prescribing information, please visit www.pfizer.com.
This Patient Information has been approved by the U.S. Food and Drug Administration.
LAB-0606-7.0
Revised November 2021
| ZMAX
azithromycin dihydrate powder, for suspension |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Labeler - Pfizer Laboratories Div Pfizer Inc (134489525) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Pfizer Ireland Pharmaceuticals | 985052076 | ANALYSIS(0069-4170) , API MANUFACTURE(0069-4170) | |