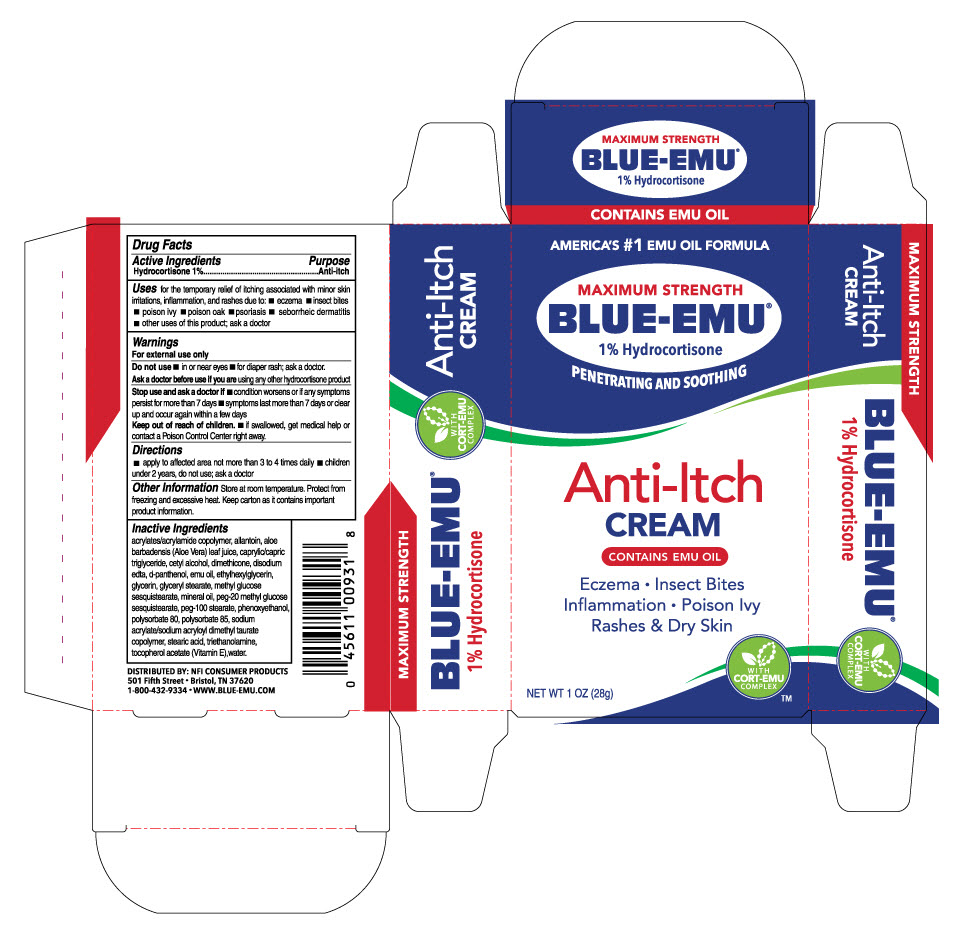
BLUE-EMU ANTI-ITCH CREAM- hydrocortisone 1% cream
Nutrition & Fitness, Inc. DBA NFI Consumer Products, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
NFI Consumer Products - Anti-Itch Cream (Hydrocortisone 1%) Drug Facts
USES
For the temporary relief of itching associated with minor skin irritations, inflammation and rashes due to:
- eczema
- insect bites
- poison ivy
- poison oak
- psoriasis
- seborrheic dermatitis
- other uses of this product should only be under the advice and supervision of a doctor
STOP USE AND ASK A DCTOR IF
- condition worsens
- symptoms persist for more than 7 days
- symptoms clear up and occur again within a few days
KEEP OUT OF THE REACH OF CHILDREN
If swallowed, get medical help or contact a Poison Control Center right away.
DIRECTIONS
- apply to affected area not more than 3 to 4 times daily
- children under 2 years, do not use; ask a doctor.
OTHER INFORMATION
Store at room temperature.
Protect from freezing and excessive heat.
Keep carton as it contains important product information.
INACTIVE INGREDIENTS
acrylates/acrylamide copolymer, allantoin, aloe barbadensis (Aloe Vera) leaf juice, caprylic/capric triglyceride, cetyl alcohol, dimethicone, disodium edta, d-panthenol, emu oil ethylhexylglycerin, glycerin, glyceryl stearate, methyl glucose sesquistearate, mineral oil, peg-20 methyl glucose sesquistearate, peg-100 stearate, phenoxyethanol, polysorbate 80, polysorbate 85, sodium acrylate/sodium acryloyl dimethyl taurate copolymer, stearic acid, triethanolamine, tocopherol acetate (Vitamin E), water.
| BLUE-EMU ANTI-ITCH CREAM
hydrocortisone 1% cream |
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Nutrition & Fitness, Inc. DBA NFI Consumer Products, Inc. (016785052) |