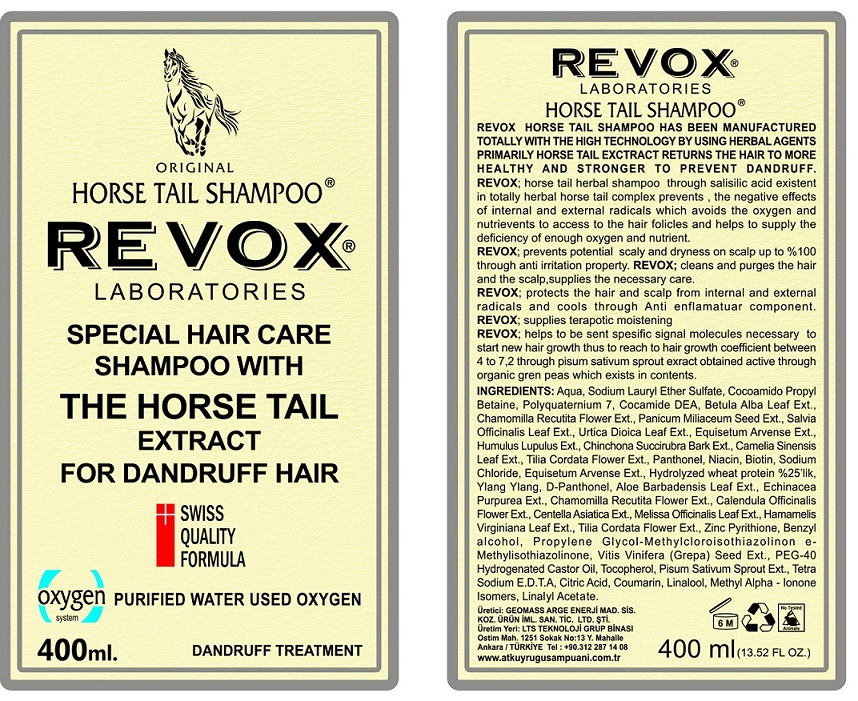
Label: REVOX- pyrithione zinc shampoo
-
Contains inactivated NDC Code(s)
NDC Code(s): 72029-001-01 - Packager: GEOMASS ARASTIRMA GELISTIRME ENERJI PETROL MADEN SIS ILAC VE KOZ URUN IM SAN TIC LTD STI
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated January 30, 2018
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- PURPOSE
- INDICATIONS & USAGE
- KEEP OUT OF REACH OF CHILDREN
- WARNINGS
- DOSAGE & ADMINISTRATION
-
Inactive Ingredients
Aqua, Sodium Lauryl Ether Sulfate, Cocoamido Propyl Betaine, Polyquarternium 7, Cocamide DEA, Betula Alba Leaf Ext., Chamomilla Recutita Flower Ext., Panicum Miliaceum Seed Ext., Salvia Officinalis Leaf Ext., Urtica Dioica Leaf Ext., Equisetum Arvense Ext., Humulus Lupulus Ext., Cinchona Succirubra Bark Ext., Camelia Sinensis Leaf Ext., Tilia Cordata Flower Ext., Panthenol, Niacin, Biotin , Sodium Chloride, Equisetum Arvense Ext., Hydrolyzed Wheat Protein, Ylang Ylang, D-Panthenol, Aloe Barbadensis Leaf Ext., Echinacea Purpurea Ext., Chamomilla Recutita Flower Ext., Calendula Officinalis Flower Ext., Centella Asiatica Ext., Melissa Officinalis Leaf Ext., Hamamelis Virginiana Leaf Ext., Tilia Cordata Flower Ext., Benzyl Alcohol, Propylene Glycol-Methylcloroisothiazolinone- Methylisothiazolinone, Vitis Vinifera (Grape) Seed Ext., PEG- 40 Hydrogenated Castor Oil, Tocopherol, Pisum Sativum Sprout Ext., Tetra Sodium E.D.T.A, Citric Acid, Coumarin, Linalool, Methyl Alpha- Ionone Isomer, Linalyl Acetate.
- Packaging
-
INGREDIENTS AND APPEARANCE
REVOX
pyrithione zinc shampooProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:72029-001 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PYRITHIONE ZINC (UNII: R953O2RHZ5) (PYRITHIONE ZINC - UNII:R953O2RHZ5) PYRITHIONE ZINC 2 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) SODIUM LAURETH-3 SULFATE (UNII: BPV390UAP0) COCAMIDOPROPYL BETAINE (UNII: 5OCF3O11KX) POLYQUATERNIUM-7 (70/30 ACRYLAMIDE/DADMAC; 1600000 MW) (UNII: 0L414VCS5Y) COCO DIETHANOLAMIDE (UNII: 92005F972D) BETULA PUBESCENS LEAF (UNII: 84SOH0O3OO) CHAMOMILE (UNII: FGL3685T2X) MILLET (UNII: TJR6B3R47P) SAGE (UNII: 065C5D077J) URTICA DIOICA LEAF (UNII: X6M0DRN46Q) EQUISETUM ARVENSE BRANCH (UNII: 1L0VKZ185E) HOPS (UNII: 01G73H6H83) CINCHONA PUBESCENS BARK (UNII: S96N10R972) GREEN TEA LEAF (UNII: W2ZU1RY8B0) TILIA CORDATA FLOWER (UNII: CFN6G1F6YK) PANTHENOL (UNII: WV9CM0O67Z) NIACIN (UNII: 2679MF687A) BIOTIN (UNII: 6SO6U10H04) SODIUM CHLORIDE (UNII: 451W47IQ8X) HYDROLYZED WHEAT PROTEIN (ENZYMATIC, 3000 MW) (UNII: J2S07SB0YL) CANANGA OIL (UNII: 8YOY78GNNX) DEXPANTHENOL (UNII: 1O6C93RI7Z) ALOE VERA LEAF (UNII: ZY81Z83H0X) ECHINACEA PURPUREA (UNII: QI7G114Y98) CALENDULA OFFICINALIS FLOWER (UNII: P0M7O4Y7YD) CENTELLA ASIATICA (UNII: 7M867G6T1U) MELISSA OFFICINALIS LEAF (UNII: 50D2ZE9219) HAMAMELIS VIRGINIANA LEAF (UNII: T07U1161SV) BENZYL ALCOHOL (UNII: LKG8494WBH) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) METHYLCHLOROISOTHIAZOLINONE (UNII: DEL7T5QRPN) METHYLISOTHIAZOLINONE (UNII: 229D0E1QFA) VITIS VINIFERA SEED (UNII: C34U15ICXA) POLYOXYL 40 HYDROGENATED CASTOR OIL (UNII: 7YC686GQ8F) TOCOPHEROL (UNII: R0ZB2556P8) PEA (UNII: W4X7H8GYFM) EDETATE SODIUM (UNII: MP1J8420LU) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) COUMARIN (UNII: A4VZ22K1WT) LINALOOL, (+/-)- (UNII: D81QY6I88E) METHYL-.ALPHA.-IONONE (UNII: QMK5X3FBG7) LINALYL ACETATE (UNII: 5K47SSQ51G) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72029-001-01 1 in 1 CARTON 01/30/2018 1 400 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part358H 01/30/2018 Labeler - GEOMASS ARASTIRMA GELISTIRME ENERJI PETROL MADEN SIS ILAC VE KOZ URUN IM SAN TIC LTD STI (505204333) Establishment Name Address ID/FEI Business Operations GEOMASS ARASTIRMA GELISTIRME ENERJI PETROL MADEN SIS ILAC VE KOZ URUN IM SAN TIC LTD STI 505204333 manufacture(72029-001)