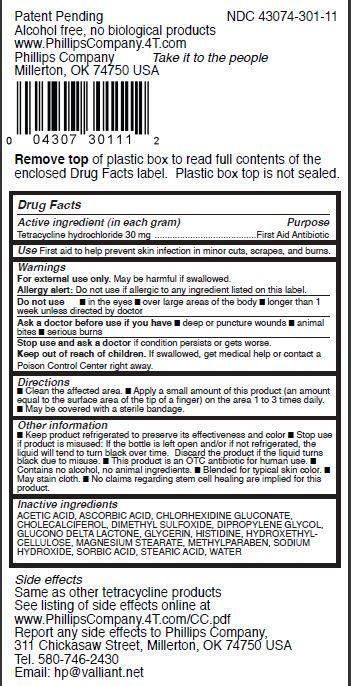
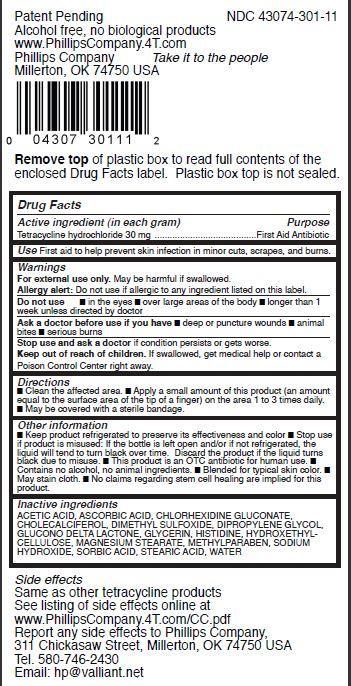
Label: TETRASTEM- tetracycline hydrochloride ointment
-
Contains inactivated NDC Code(s)
NDC Code(s): 43074-301-11 - Packager: Phillips Company
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated February 25, 2014
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredient (in each gram)
- Purpose
- Use
- Warnings
- Directions
-
Other information
- Keep product refrigerated to preserve its effectiveness and color
- Stop use if product is misused: If the bottle is left open and/or if not refrigerated, the liquid will tend to turn black over time. Discard the product if the liquid turns black due to misuse.
- This product is an OTC antibiotic for human use.
- Contains no alcohol, no animal ingredients.
- Blended for typical skin color.
- May stain cloth.
- No claims regarding stem cell healing are implied for this product.
- Inactive ingredients
-
Package Label
TetraStem
brand
Refrigerate
Topical
Ointment
First Aid
Antibiotic
Tetracycline
Hydrochloride
NET WT. 0.1 OZ (3 G)Patent Pending NDC 43074-301-11
Alcohol free, no biological products
www.PhillipsCompany.4T.com
Phillips Company Take it to the people
Millerton, OK 74750 USA
Remove top of plastic box to read full contents of the
enclosed Drug Facts label. Plastic box top is not sealed.Side effects
Same as other tetracycline products
See listing of side effects online at
www.PhillipsCompany.4T.com/CC.pdf
Report any side effects to Phillips Company,
311 Chickasaw Street, Millerton, OK 74750 USA
Tel. 580-746-2430
Email: hp@valliant.net TetraStem
-
INGREDIENTS AND APPEARANCE
TETRASTEM
tetracycline hydrochloride ointmentProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:43074-301 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TETRACYCLINE HYDROCHLORIDE (UNII: P6R62377KV) (TETRACYCLINE - UNII:F8VB5M810T) TETRACYCLINE HYDROCHLORIDE 30 mg in 1 g Inactive Ingredients Ingredient Name Strength ACETIC ACID (UNII: Q40Q9N063P) ASCORBIC ACID (UNII: PQ6CK8PD0R) CHLORHEXIDINE GLUCONATE (UNII: MOR84MUD8E) CHOLECALCIFEROL (UNII: 1C6V77QF41) DIMETHYL SULFOXIDE (UNII: YOW8V9698H) DIPROPYLENE GLYCOL (UNII: E107L85C40) GLUCONOLACTONE (UNII: WQ29KQ9POT) GLYCERIN (UNII: PDC6A3C0OX) HISTIDINE (UNII: 4QD397987E) MAGNESIUM STEARATE (UNII: 70097M6I30) METHYLPARABEN (UNII: A2I8C7HI9T) SODIUM HYDROXIDE (UNII: 55X04QC32I) SORBIC ACID (UNII: X045WJ989B) STEARIC ACID (UNII: 4ELV7Z65AP) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:43074-301-11 3 g in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part333B 02/25/2014 Labeler - Phillips Company (612368238) Establishment Name Address ID/FEI Business Operations Phillips Company 612368238 manufacture(43074-301)