GMC MEDICAL ACNE TREATING- salicylic acid gel
Laboratoires dermo Cosmetik Inc
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
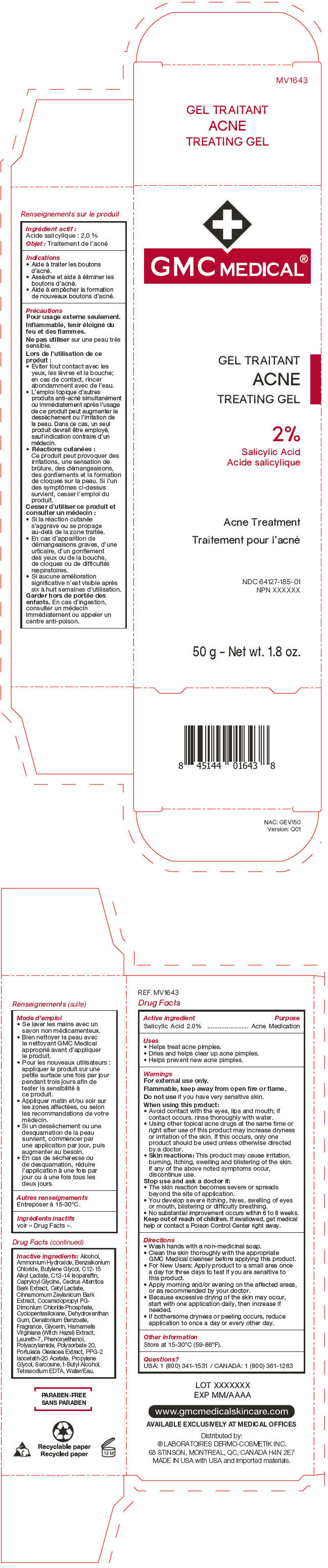
GMC Medical®
Acne Treating Gel
Uses
- Helps treat acne pimples.
- Dries and helps clear up acne pimples.
- Helps prevent new acne pimples.
Warnings
For external use only.
Flammable, keep away from open fire or flame.
When using this product
- Avoid contact with the eyes, lips and mouth; if contact occurs, rinse thoroughly with water.
- Using other topical acne drugs at the same time or right after use of this product may increase dryness or irritation of the skin. If this occurs, only one product should be used unless otherwise directed by a doctor.
- Skin reactions: This product may cause irritation, burning, itching, swelling and blistering of the skin. If any of the above noted symptoms occur, discontinue use.
Directions
- Wash hands with a non-medicinal soap.
- Clean the skin thoroughly with the appropriate GMC Medical cleanser before applying this product.
- For New Users: Apply product to a small area once a day for three days to test if you are sensitive to this product.
- Apply morning and/or evening on the affected areas, or as recommended by your doctor.
- Because excessive drying of the skin may occur, start with one application daily, then increase if needed.
- If bothersome dryness or peeling occurs, reduce application to once a day or every other day.
Inactive ingredients
Alcohol, Ammonium Hydroxide, Benzalkonium Chloride, Butylene Glycol, C12-15 Alkyl Lactate, C13-14 Isoparaffin, Capryloyl Glycine, Cedrus Atlantica Bark Extract, Cetyl Lactate, Cinnamomum Zeylanicum Bark Extract, Cocamidopropyl PG-Dimonium Chloride Phosphate, Cyclopentasiloxane, Dehydroxanthan Gum, Denatonium Benzoate, Fragrance, Glycerin, Hamamelis Virginiana (Witch Hazel) Extract, Laureth-7, Phenoxyethanol, Polyacrylamide, Polysorbate 20, Portulaca Oleracea Extract, PPG-2 Isoceteth-20 Acetate, Propylene Glycol, Sarcosine, t-Butyl Alcohol, Tetrasodium EDTA, Water/Eau.
| GMC MEDICAL
ACNE TREATING
salicylic acid gel |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Laboratoires dermo Cosmetik Inc (249335480) |