MEDIHALER-EPI- epinephrine bitartrate aerosol
3M Company
----------
Medihaler-Epi™
Epinephrine Bitartrate Inhalation Aerosol
Bronchodilator
INDICATIONS
For temporary relief of shortness of breath, tightness of chest and wheezing due to bronchial asthma. Eases breathing for asthma patients by reducing spasms of bronchial muscles. For the symptomatic control of bronchial asthma.
CONTAINS
Active ingredient: epinephrine bitartrate, 7.0 mg per mL. Inactive ingredients: cetylpyridinium chloride, dichlorodifluoromethane, dichlorotetrafluoroethane, sorbitan trioleate, and trichloromonofluoromethane.
WARNING
Contains dichlorodifluoromethane, dichlorotetrafluoroethane and trichloromonofluoromethane, substances which harm public health and environment by destroying ozone in the upper atmosphere.
FOR ORAL INHALATION THERAPY ONLY
DIRECTIONS
Each inhalation contains the equivalent of 0.16 milligrams epinephrine delivered at the mouthpiece. Inhalation dosage for adults and children 4 years of age and older: start with one inhalation, then wait at least one minute. If not relieved, use MEDIHALER-EPI once more. Do not use again for at least three hours. The use of this product by children should be supervised by an adult. Children under 4 years of age: consult a physician.
MEDIHALER-EPI delivers the same dose every time. It does not depend on the strength with which the canister is pressed against the oral adapter or on the time the canister is held down. The canister must be released before it can be depressed again for another dose.
MEDIHALER-EPI is designed to deliver a sufficient dose for most people in one or occasionally two inhalations. It is not necessary to use several applications in rapid succession. Use only when you actually need relief. Do no get into the habit of using MEDIHALER-EPI indiscriminately.
Before each use, remove dust cap and inspect oral adapter for foreign objects. Shake MADIHALER-EPI well. To get the medication deep into the lungs, follow these three simple steps which can be completed within five seconds:
- Breath out fully and place mouthpiece well into the mouth, aimed at the back of the throat.
- As you begin to breathe in deeply, press the canister firmly down into the adapter with the index finger, and continue to inhale. This releases one dose.
- Release pressure on canister and remove unit from the mouth. Hold the breath as long as possible, then breathe out slowly.
Replace dust cap after each use.
MEDIHALER-EPI is packaged in a shatterproof aluminum canister fitted with a specially designed valve. It can be used only with the MEDIHALER-EPI oral adapter, a non-breakable inhaler device.
WARNINGS
Do not use this product unless a diagnosis of asthma has been made by a physician. Do not use this product if you have heart disease, high blood pressure, thyroid disease, diabetes or difficulty in urination due to enlargement of the prostate gland, unless directed by a physician. Do not use this product if you have ever been hospitalized for asthma or if you are taking any prescription drug for asthma unless directed by a physician.
MEDIHALER-EPI is intended for oral inhalation only. Do not use this product more frequently or at higher doses than recommended unless directed by a physician. Excessive use may cause nervousness and rapid heartbeat, and, possibly adverse effects on the heart. Do not continue to use this product, but seek medical assistance immediately if symptoms are not relieved within 20 minutes or become worse.
As with any drug, if you are pregnant or nursing a baby, seek the advise of a health professional before using this product. Keep this and all drugs out of the reach of children. In case of accidental overdose, seek professional assistance or contact a poison control center immediately.
DRUG INTERACTION PRECAUTION
Do not use this product if you are presently taking a prescription drug for high blood pressure or depression without first consulting your physician.
CONTENTS UNDER PRESSURE
Do not puncture or incinerate canister. Do not store near heat. Exposure to temperatures above 120˚F may cause bursting. Store at controlled room temperature between 15˚C and 30˚C (59˚F and 86˚F).
DESCRIPTION
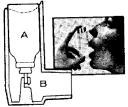
For speed of relief and convenience, MEDIHALER-EPI oral inhaler comes ready to use. It consists of two parts:
A. An aluminum canister, containing the medication, with a single dose valve, which fits into
B. The white plastic oral adapter. The canister can be used only with the MEDIHALER-EPI oral adapter.
The plastic oral adapter should be cleaned daily. Simply remove canister, wash adapter with soap and hot water, and rinse thoroughly. Dry adapter and replace the canister and dust cap.
AVAILABILITY
15 mL size: available as a combination package (canister with plastic oral adapter) or as a refill (canister only), each canister contains a minimum of 300 metered inhalations.
3M
3M Pharmaceuticals
Northridge, CA 91324
EPI-17 JUNE 1993
| MEDIHALER-EPI
epinephrine bitartrate aerosol |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - 3M Company (006173082) |