SLEEP TABS- diphenhydramine hcl tablet
L&R Distributors, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Select Brand 44-189-Discontinued
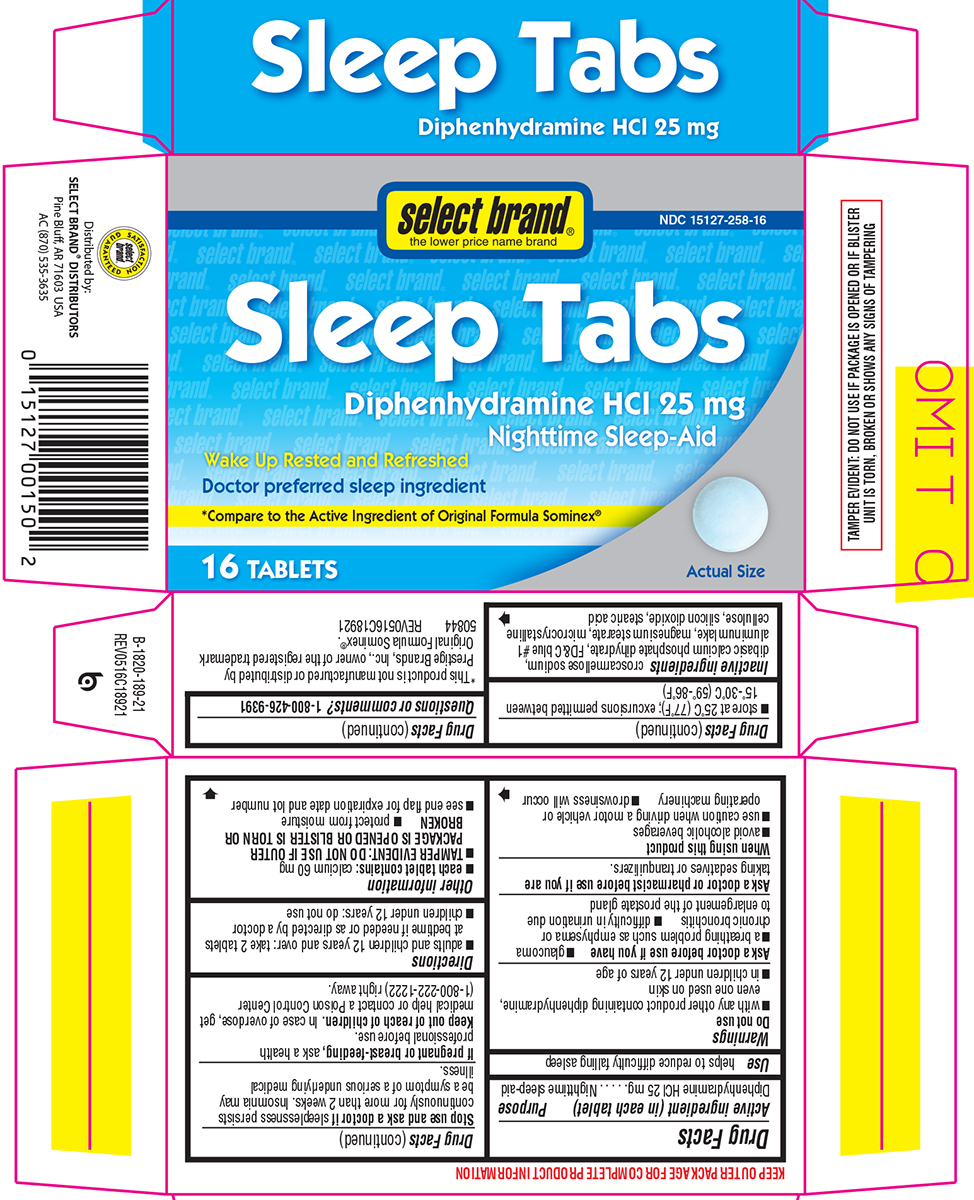
Warnings
Do not use
- with any other product containing diphenhydramine, even one used on skin
- in children under 12 years of age
Ask a doctor before use if you have
- glaucoma
- a breathing problem such as emphysema or chronic bronchitis
- difficulty in urination due to enlargement of the prostate gland
When using this product
- avoid alcoholic beverages
- use caution when driving a motor vehicle or operating machinery
- drowsiness will occur
Directions
- adults and children 12 years and over: take 2 tablets at bedtime if needed or as directed by a doctor
- children under 12 years: do not use
Other information
- each tablet contains: calcium 60 mg
- TAMPER EVIDENT: DO NOT USE IF OUTER PACKAGE IS OPENED OR BLISTER IS TORN OR BROKEN
- protect from moisture
- store at 25ºC (77ºF); excursions permitted between 15º-30ºC (59º-86ºF)
- see end flap for expiration date and lot number
Inactive ingredients
croscarmellose sodium, dibasic calcium phosphate dihydrate, FD&C blue #1 aluminum lake, magnesium stearate, microcrystalline cellulose, silicon dioxide, stearic acid
Principal Display Panel
select brand®
the lower price name brand
NDC 15127-258-16
Sleep Tabs
Diphenhydramine HCl 25 mg
Nighttime Sleep-Aid
Wake Up Rested and Refreshed
Doctor preferred sleep ingredient
*Compare to the Active Ingredient of Original Formula Sominex®
16 TABLETS
Actual Size
TAMPER EVIDENT: DO NOT USE IF PACKAGE IS OPENED OR IF BLISTER
UNIT IS TORN, BROKEN OR SHOWS ANY SIGNS OF TAMPERING
*This product is not manufactured or distributed by
Prestige Brands, Inc., owner of the registered trademark
Original Formula Sominex®.
50844 REV0516C18921
Distributed by:
SELECT BRAND® DISTRIBUTORS
Pine Bluff, AR 71603 USA
AC (870) 535-3635
SATISFACTION GUARANTEED
Select Brand 44-189
| SLEEP TABS
diphenhydramine hcl tablet |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - L&R Distributors, Inc. (012578514) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 832867894 | MANUFACTURE(15127-258) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 038154464 | PACK(15127-258) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 832867837 | PACK(15127-258) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 967626305 | PACK(15127-258) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 868734088 | PACK(15127-258) | |