Label: MIPASTE PLUS STRAWBERRY- sodium fluoride paste, dentifrice
MIPASTE PLUS- sodium fluoride paste, dentifrice
-
NDC Code(s):
61596-880-40,
61596-880-41,
61596-882-40,
61596-882-41, view more61596-884-40, 61596-884-41, 61596-888-40, 61596-888-41, 61596-986-40, 61596-986-41
- Packager: GC America Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated January 30, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Use
- Warnings
- Warnings
-
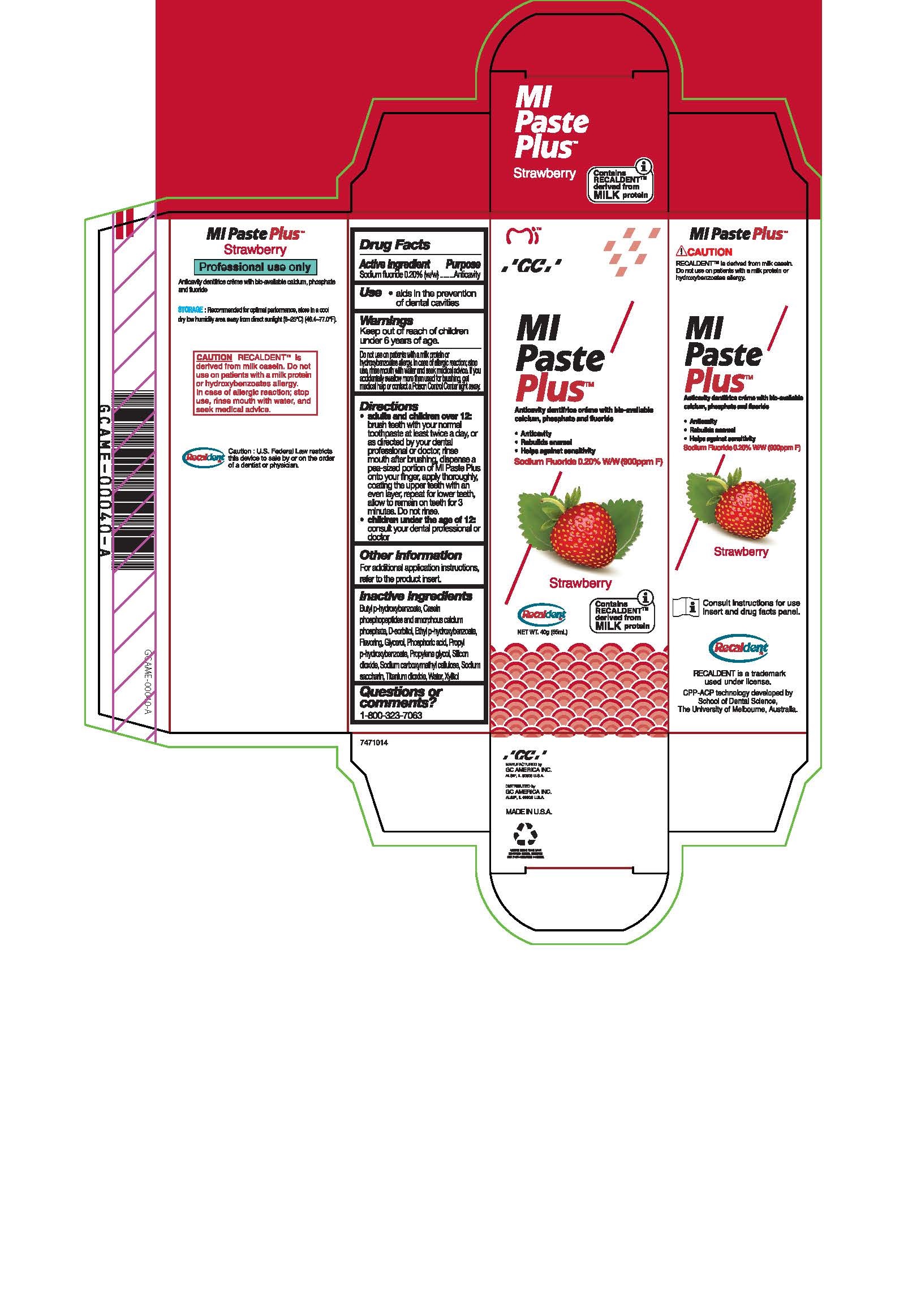
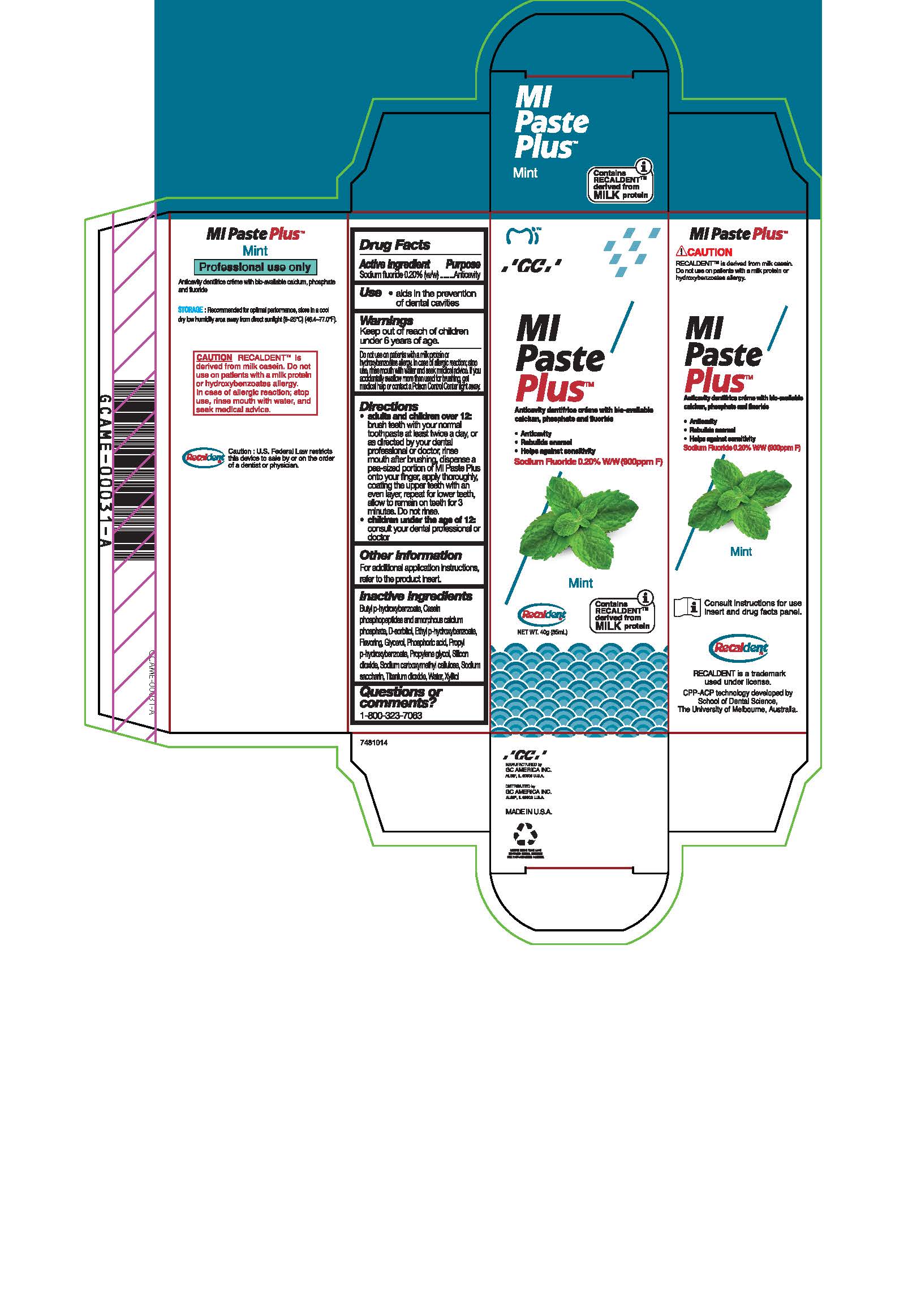
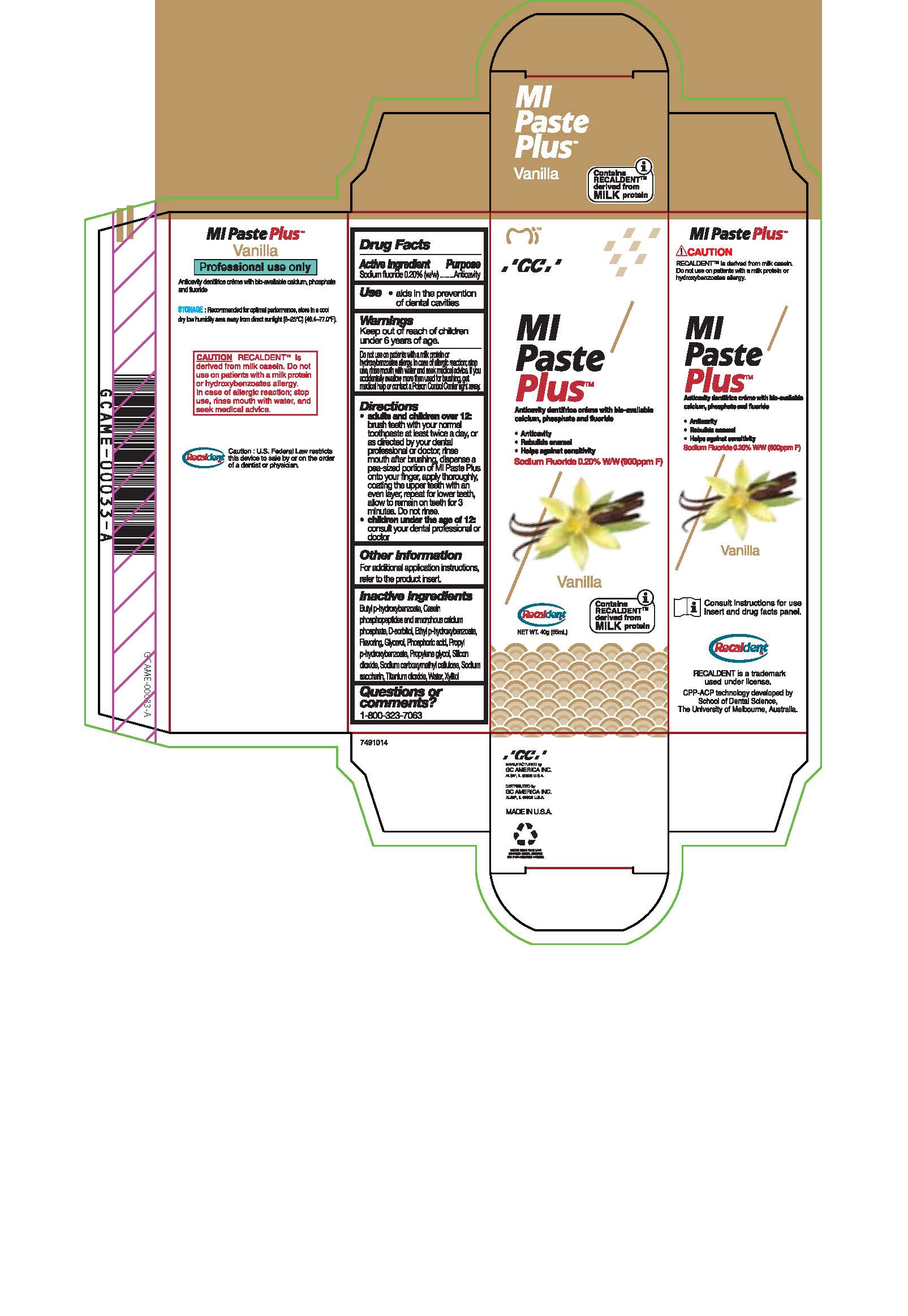
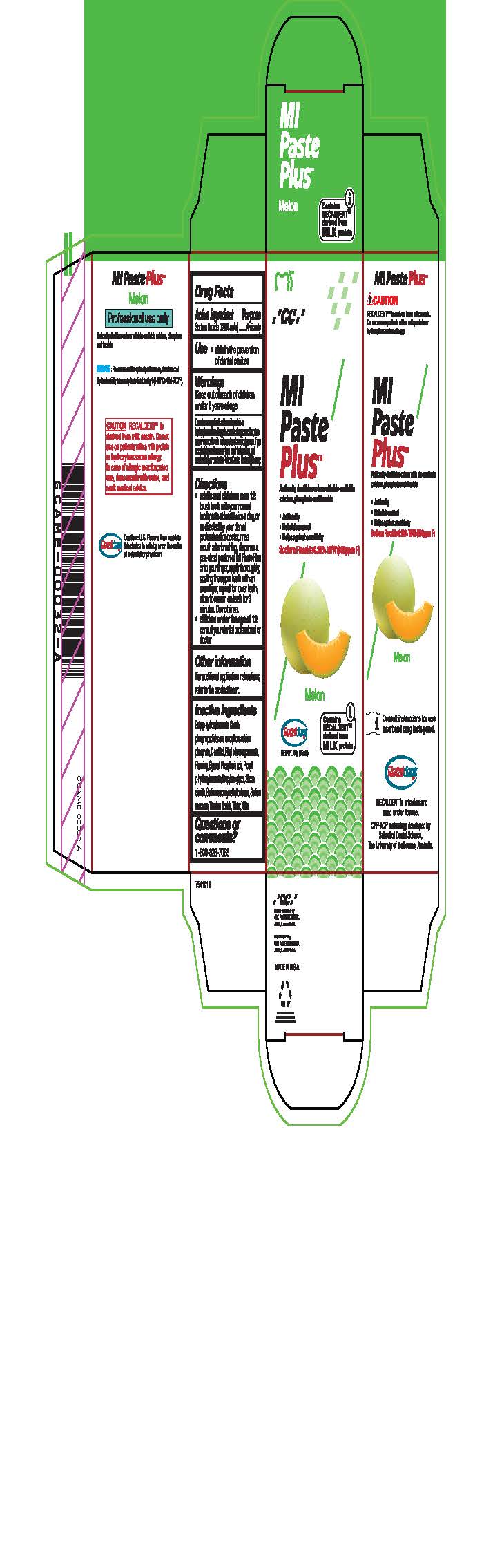
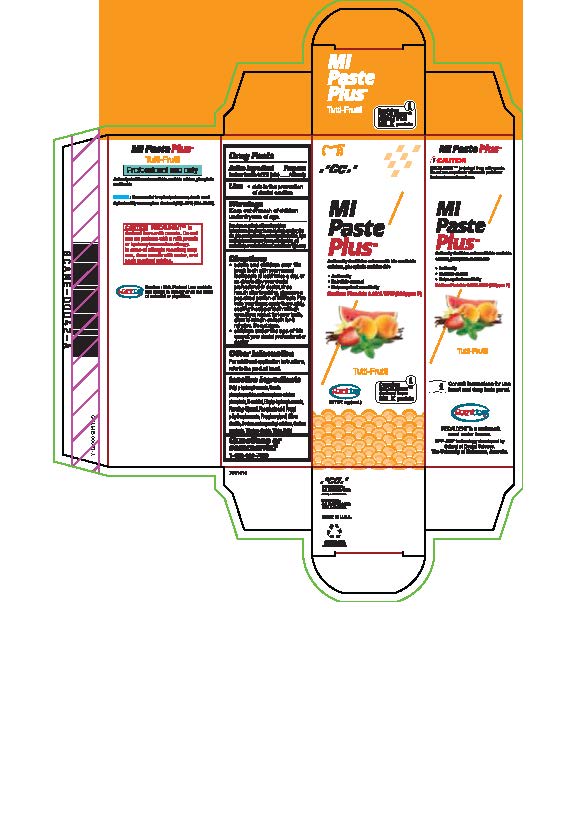
Directions
* Adults and children over 12: brush teeth with your normal toothpaste at least twice a day, or as directed by your dental profession or doctor, rinse mouth after brushing, dispense a pea-sized portion of MI Paste Plus onto your finger, apply thoroughly, coating the upper teeth with an even layer, repeat for lower teeth, allow to remain on teeth for 3 mintues. Do not rinse.
* Children under the age of 12: consult your dental professional or doctor.
- Other information
- Inactive ingredients
- Questions or comments?
- Recommended Indications
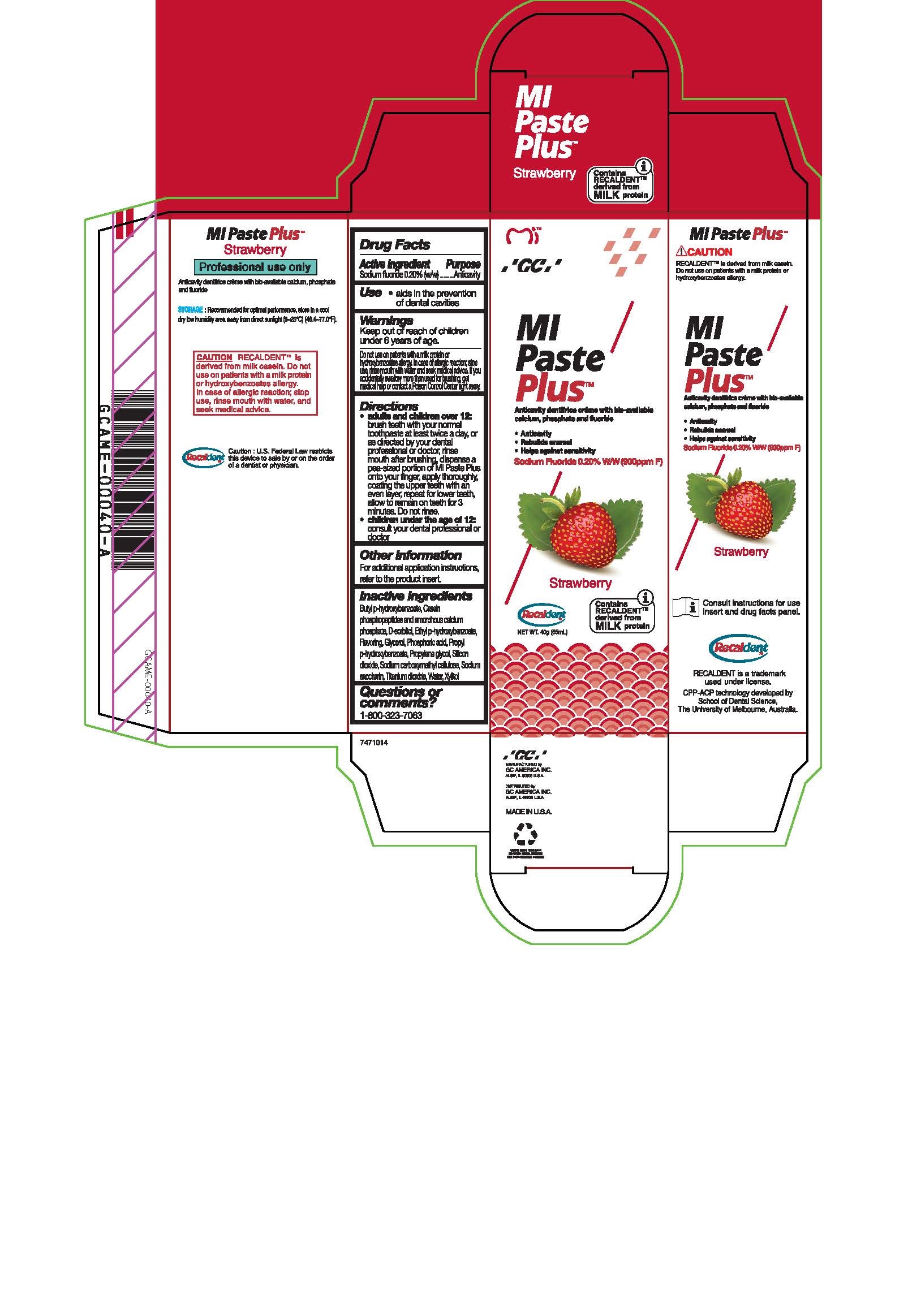
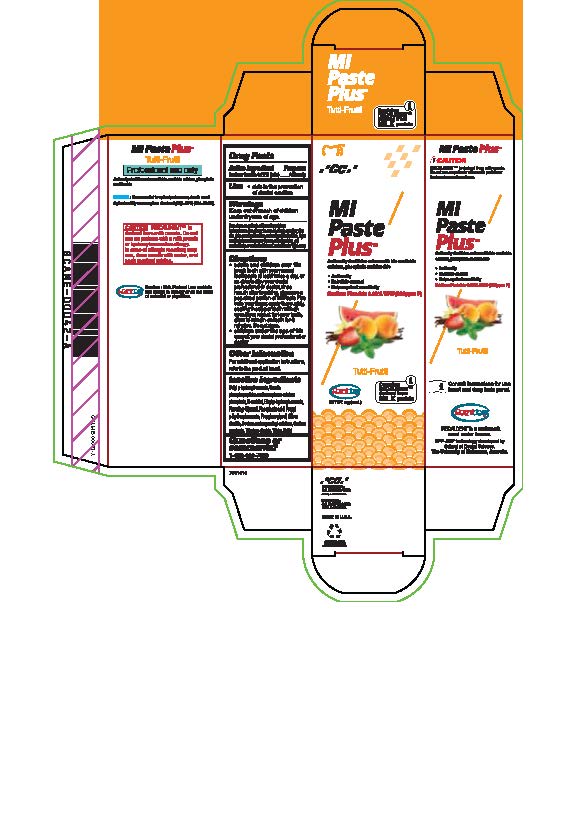
- Tube Box Strawberry
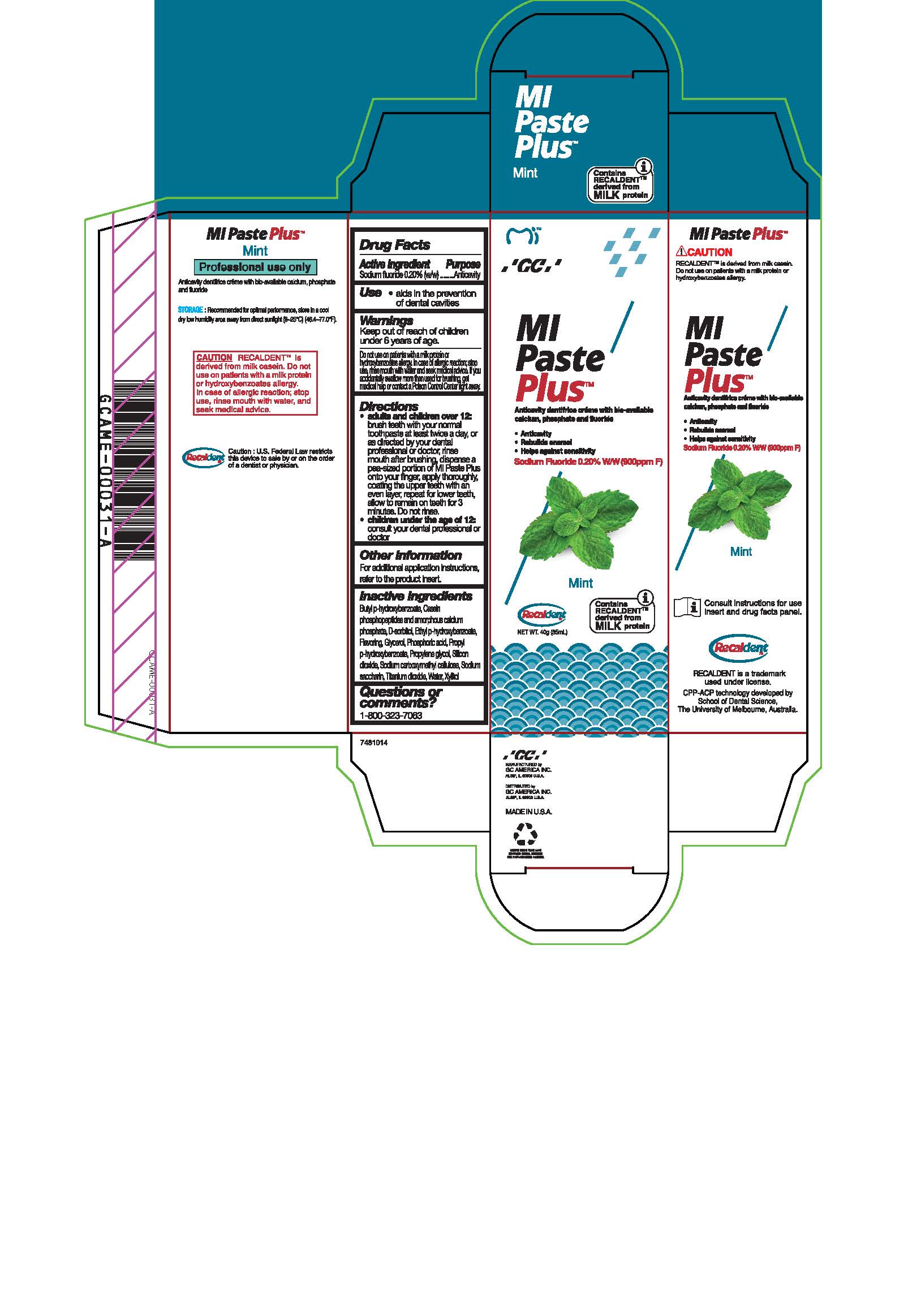
- Tube Box Mint
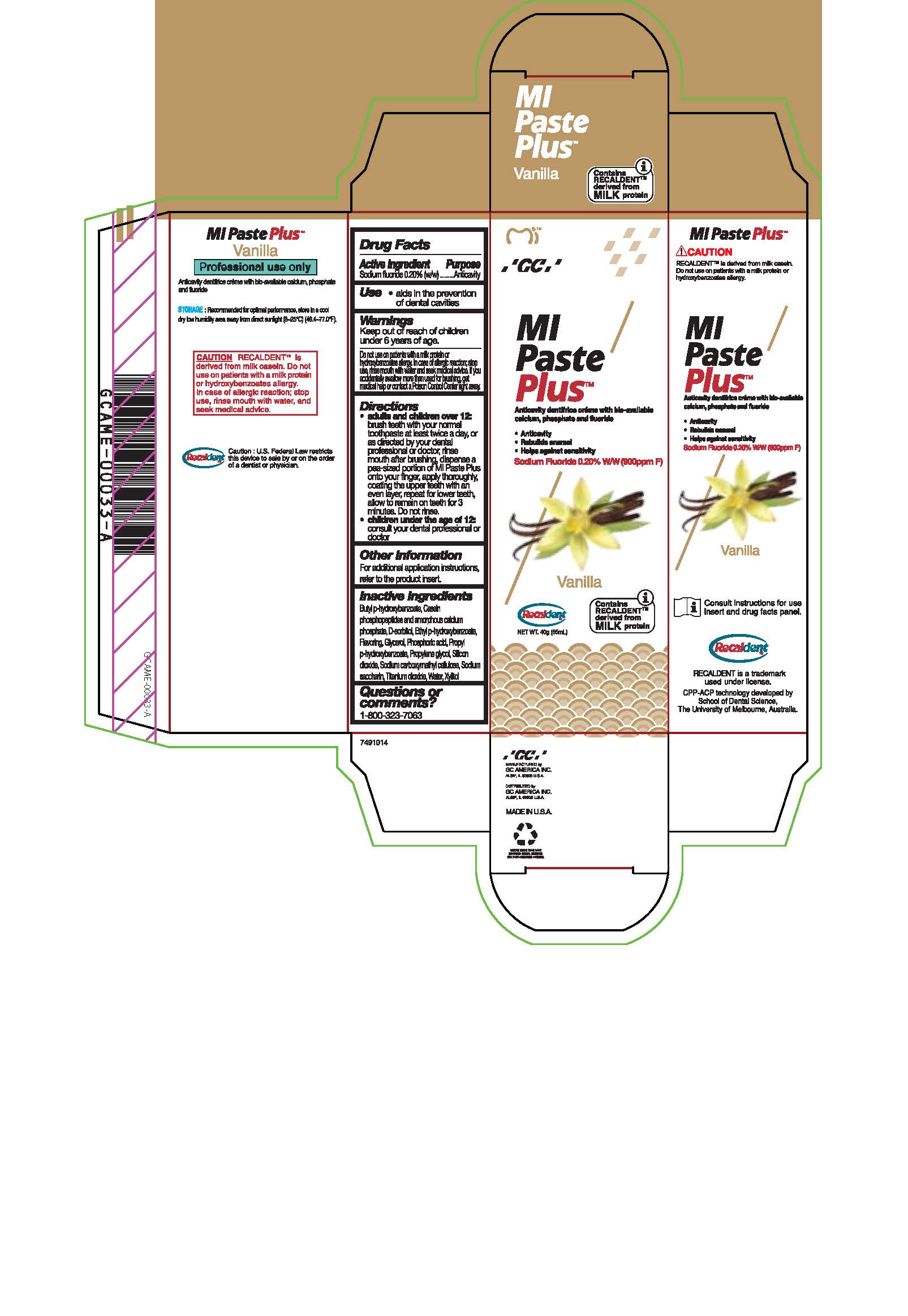
- Tube Box Vanilla
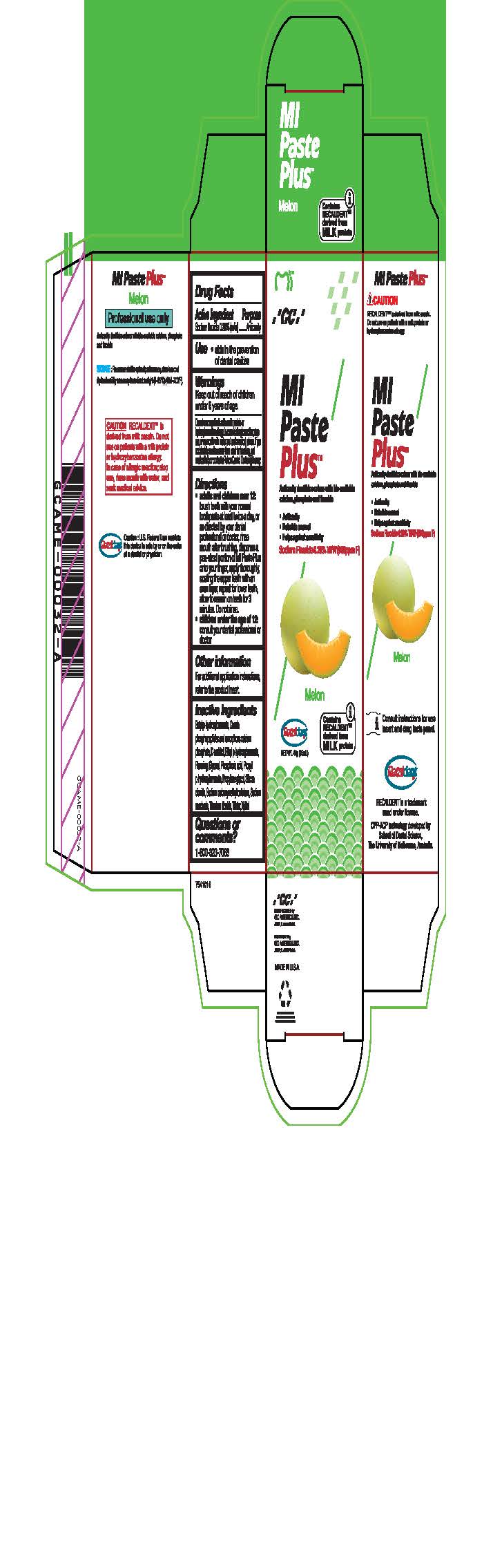
- Tube Box Melon
- Tube box Tutti-Frutti
-
INGREDIENTS AND APPEARANCE
MIPASTE PLUS STRAWBERRY
sodium fluoride paste, dentifriceProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:61596-986 Route of Administration DENTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM FLUORIDE (UNII: 8ZYQ1474W7) (FLUORIDE ION - UNII:Q80VPU408O) SODIUM FLUORIDE 0.2 g in 100 g Inactive Ingredients Ingredient Name Strength CARBOXYMETHYLCELLULOSE SODIUM, UNSPECIFIED FORM (UNII: K679OBS311) SORBITOL (UNII: 506T60A25R) WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) PROPYLPARABEN (UNII: Z8IX2SC1OH) PHOSPHORIC ACID (UNII: E4GA8884NN) SACCHARIN SODIUM (UNII: SB8ZUX40TY) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) ETHYLPARABEN (UNII: 14255EXE39) CALCIUM PHOSPHATE (UNII: 97Z1WI3NDX) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) XYLITOL (UNII: VCQ006KQ1E) BUTYLPARABEN (UNII: 3QPI1U3FV8) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) Product Characteristics Color Score Shape Size Flavor STRAWBERRY Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:61596-986-41 1 in 1 BOX 06/01/2015 1 NDC:61596-986-40 40 g in 1 TUBE; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) 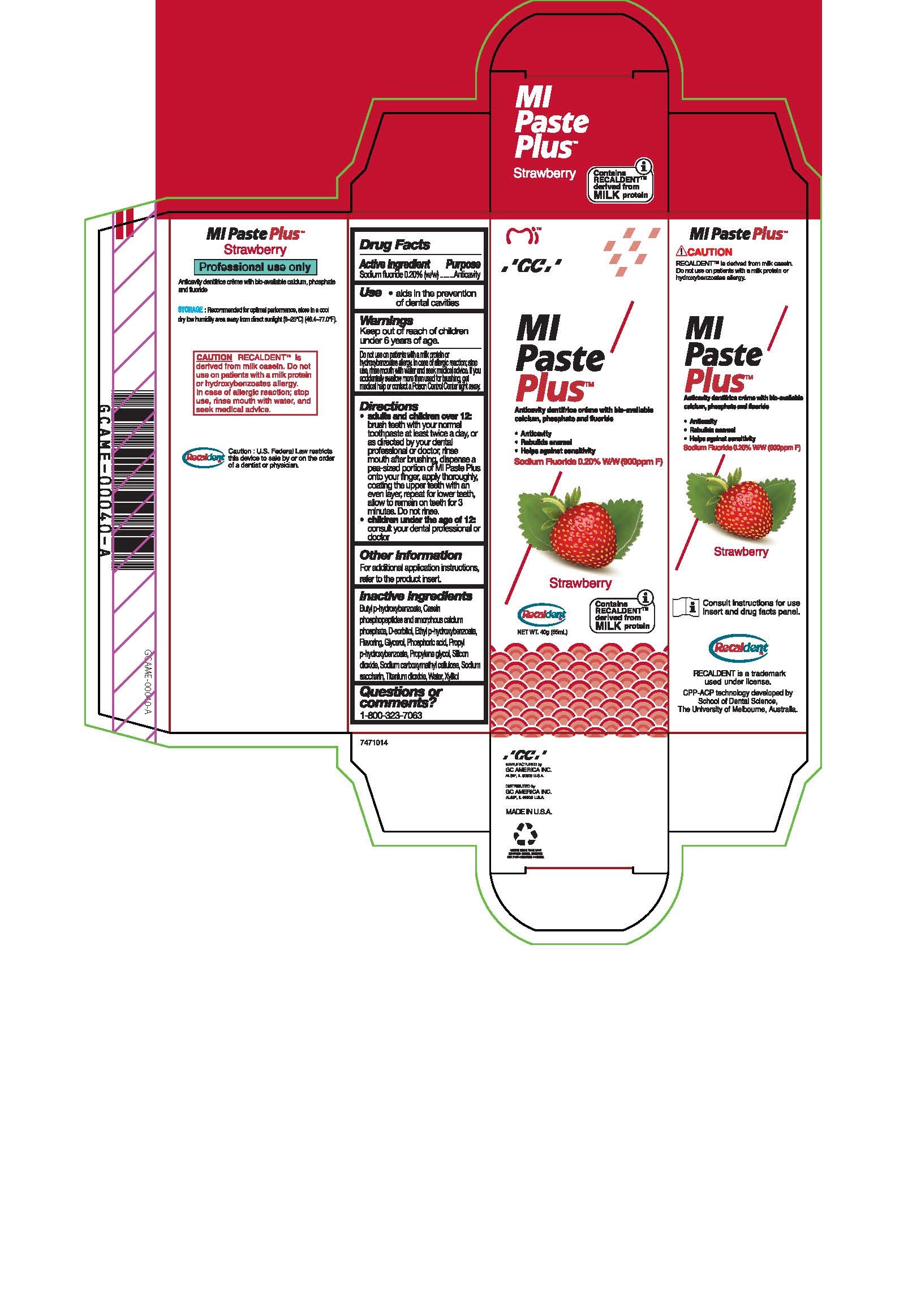
Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M021 06/01/2015 MIPASTE PLUS
sodium fluoride paste, dentifriceProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:61596-884 Route of Administration DENTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM FLUORIDE (UNII: 8ZYQ1474W7) (FLUORIDE ION - UNII:Q80VPU408O) SODIUM FLUORIDE 0.2 g in 100 g Inactive Ingredients Ingredient Name Strength XYLITOL (UNII: VCQ006KQ1E) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) CARBOXYMETHYLCELLULOSE SODIUM (UNII: K679OBS311) SORBITOL (UNII: 506T60A25R) WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) PROPYLPARABEN (UNII: Z8IX2SC1OH) PHOSPHORIC ACID (UNII: E4GA8884NN) SACCHARIN SODIUM (UNII: SB8ZUX40TY) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) ETHYLPARABEN (UNII: 14255EXE39) CALCIUM PHOSPHATE (UNII: 97Z1WI3NDX) BUTYLPARABEN (UNII: 3QPI1U3FV8) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) Product Characteristics Color Score Shape Size Flavor MINT Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:61596-884-41 1 in 1 BOX 06/01/2015 1 NDC:61596-884-40 40 g in 1 TUBE; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) 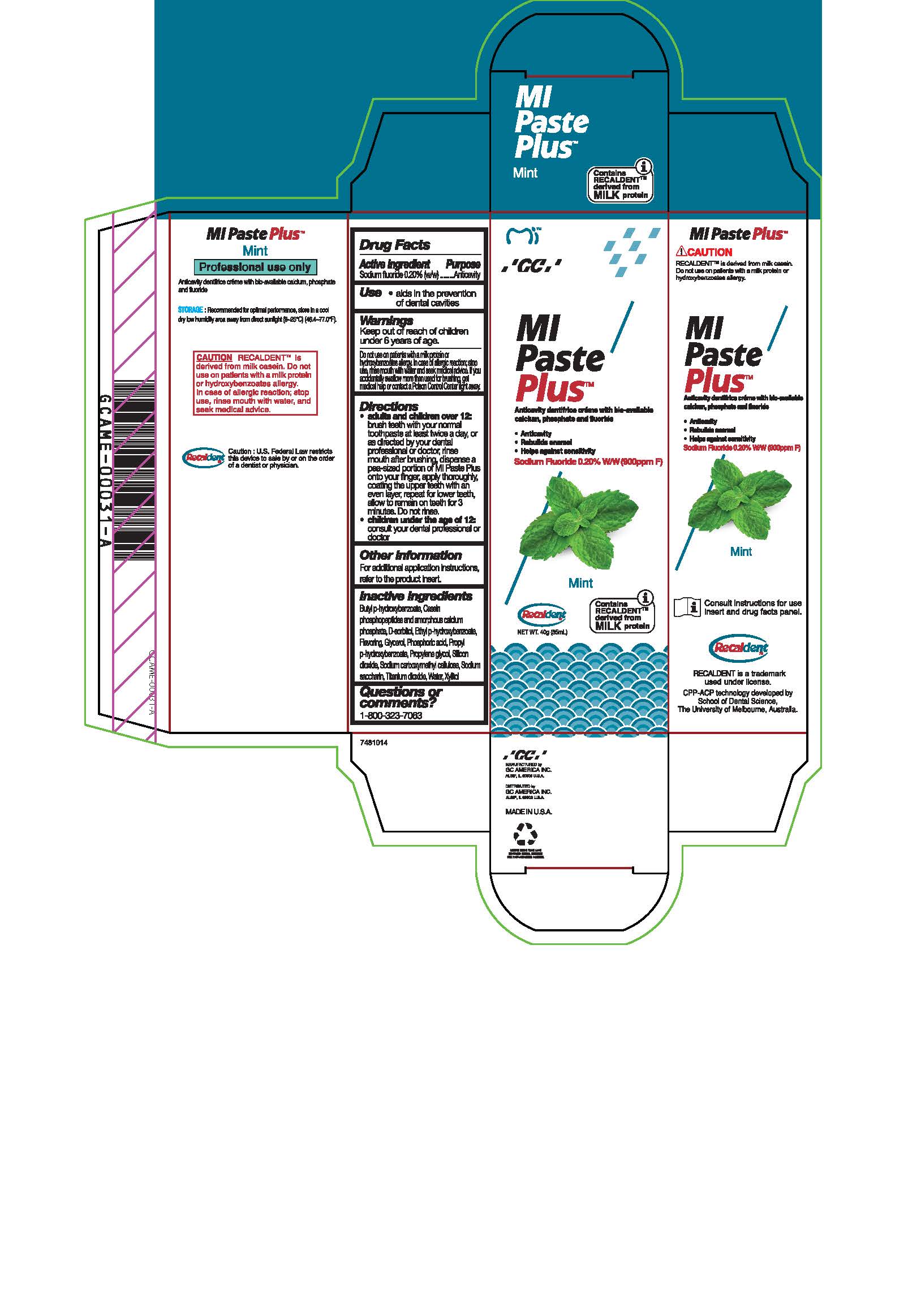
Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M021 06/01/2015 MIPASTE PLUS
sodium fluoride paste, dentifriceProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:61596-888 Route of Administration DENTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM FLUORIDE (UNII: 8ZYQ1474W7) (FLUORIDE ION - UNII:Q80VPU408O) SODIUM FLUORIDE 0.2 g in 100 g Inactive Ingredients Ingredient Name Strength PROPYLPARABEN (UNII: Z8IX2SC1OH) WATER (UNII: 059QF0KO0R) BUTYLPARABEN (UNII: 3QPI1U3FV8) SORBITOL (UNII: 506T60A25R) GLYCERIN (UNII: PDC6A3C0OX) CALCIUM PHOSPHATE (UNII: 97Z1WI3NDX) CARBOXYMETHYLCELLULOSE SODIUM (UNII: K679OBS311) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) XYLITOL (UNII: VCQ006KQ1E) PHOSPHORIC ACID (UNII: E4GA8884NN) SACCHARIN SODIUM (UNII: SB8ZUX40TY) ETHYLPARABEN (UNII: 14255EXE39) Product Characteristics Color Score Shape Size Flavor VANILLA Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:61596-888-41 1 in 1 BOX 06/01/2015 1 NDC:61596-888-40 40 g in 1 TUBE; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) 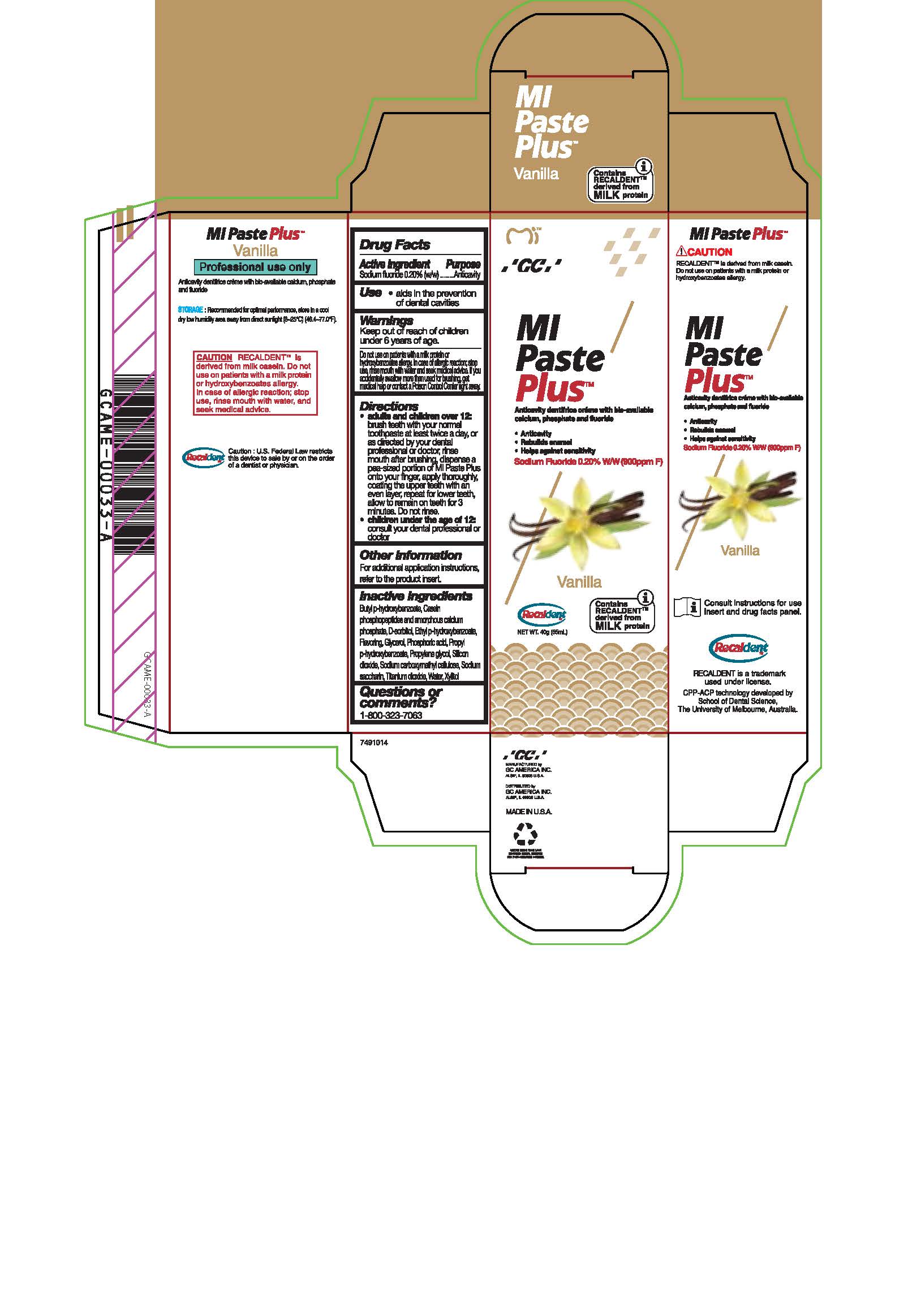
Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M021 06/01/2015 MIPASTE PLUS
sodium fluoride paste, dentifriceProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:61596-880 Route of Administration DENTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM FLUORIDE (UNII: 8ZYQ1474W7) (FLUORIDE ION - UNII:Q80VPU408O) SODIUM FLUORIDE 0.2 g in 100 g Inactive Ingredients Ingredient Name Strength SORBITOL (UNII: 506T60A25R) WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) PHOSPHORIC ACID (UNII: E4GA8884NN) BUTYLPARABEN (UNII: 3QPI1U3FV8) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SACCHARIN SODIUM (UNII: SB8ZUX40TY) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) ETHYLPARABEN (UNII: 14255EXE39) CALCIUM PHOSPHATE (UNII: 97Z1WI3NDX) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) XYLITOL (UNII: VCQ006KQ1E) CARBOXYMETHYLCELLULOSE SODIUM (UNII: K679OBS311) PROPYLPARABEN (UNII: Z8IX2SC1OH) Product Characteristics Color Score Shape Size Flavor TUTTI FRUTTI Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:61596-880-41 1 in 1 BOX 06/01/2015 1 NDC:61596-880-40 40 g in 1 TUBE; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) 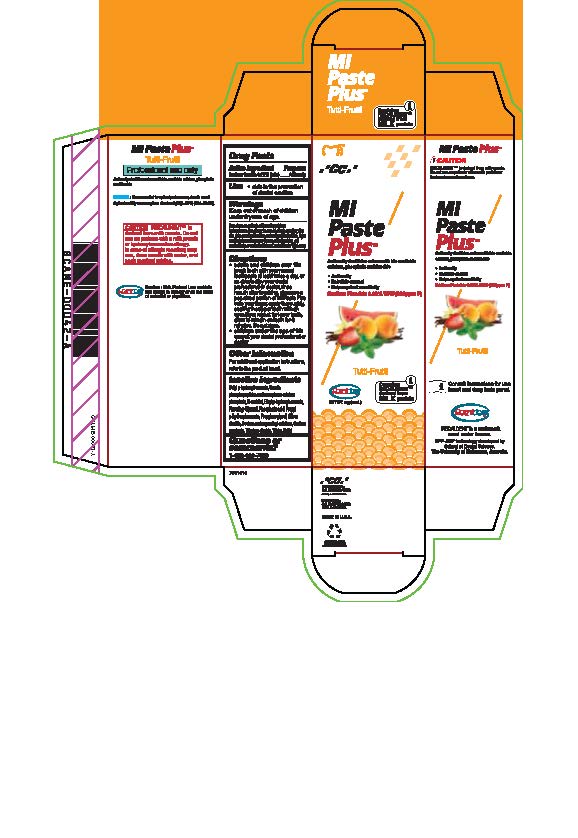
Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M021 06/01/2015 MIPASTE PLUS
sodium fluoride paste, dentifriceProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:61596-882 Route of Administration DENTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM FLUORIDE (UNII: 8ZYQ1474W7) (FLUORIDE ION - UNII:Q80VPU408O) SODIUM FLUORIDE 0.2 g in 100 g Inactive Ingredients Ingredient Name Strength SORBITOL (UNII: 506T60A25R) WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) PROPYLPARABEN (UNII: Z8IX2SC1OH) PHOSPHORIC ACID (UNII: E4GA8884NN) SACCHARIN SODIUM (UNII: SB8ZUX40TY) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) ETHYLPARABEN (UNII: 14255EXE39) CALCIUM PHOSPHATE (UNII: 97Z1WI3NDX) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) XYLITOL (UNII: VCQ006KQ1E) BUTYLPARABEN (UNII: 3QPI1U3FV8) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CARBOXYMETHYLCELLULOSE SODIUM (UNII: K679OBS311) Product Characteristics Color Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:61596-882-41 1 in 1 BOX 06/01/2015 1 NDC:61596-882-40 40 g in 1 TUBE; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) 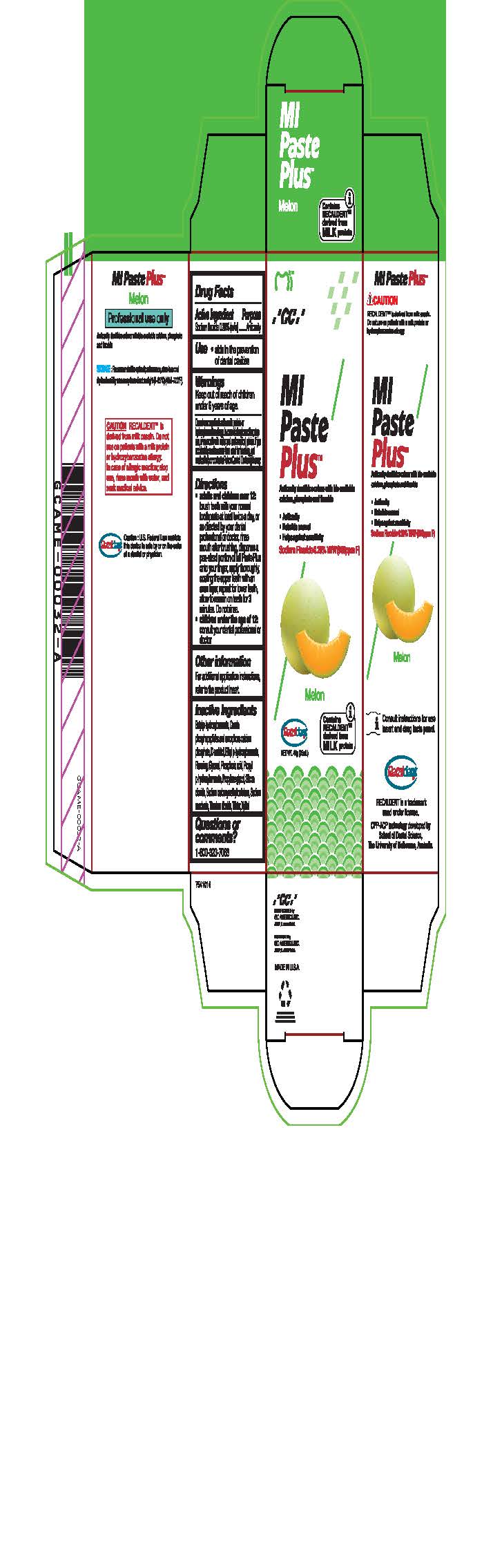
Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M021 06/01/2015 Labeler - GC America Inc. (005473608) Registrant - GC America Inc. (005473608) Establishment Name Address ID/FEI Business Operations GC America Inc. 005473608 manufacture(61596-986, 61596-884, 61596-888, 61596-880, 61596-882)