Label: ESTROPLAN- cloprostenol sodium injection, solution
- NDC Code(s): 68504-001-01, 68504-001-02
- Packager: Parnell Technologies Pty Ltd
- Category: PRESCRIPTION ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Animal Drug Application
Drug Label Information
Updated February 1, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
Approved by FDA under ANADA # 200-310
estroPLAN
(cloprostenol sodium)
Prostaglandin Analogue For Cattle
Equivalent to 250 mcg cloprostenol/mL
CAUTION: Federal law restricts this drug to use by or on the order of a licensed veterinarian.
DESCRIPTION:
estroPLAN (cloprostenol sodium) is a synthetic prostaglandin analogue structurally related to prostaglandin F2α (PGF2α). Each mL of the colorless aqueous solution contains 263 mcg of cloprostenol sodium (equivalent to 250 mcg of cloprostenol), chlorocresol 1.0 mg as a bactericide, citric acid anhydrous 0.66 mg, sodium citrate 5.03 mg, sodium chloride 6.76 mg. The pH is adjusted, as necessary, with sodium hydroxide or citric acid.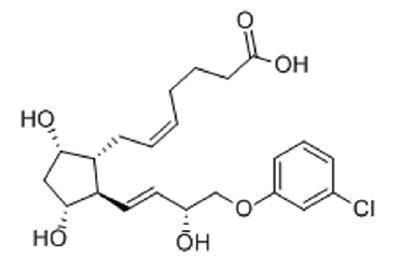
ACTION:
estroPLAN causes functional and morphological regression of the corpus luteum (luteolysis) in cattle. In normal, nonpregnant cycling animals this effect on the life span of the corpus luteum usually results in estrus 2 to 5 days after treatment. In animals with prolonged luteal function (pyometra, mummified fetus, and luteal cysts), the induced luteolysis usually results in resolution of the condition and return to cyclicity. Pregnant animals may abort depending on the stage of gestation.INDICATIONS:
For intramuscular use to induce luteolysis in beef and dairy cattle. The luteolytic action of estroPLAN can be utilized to manipulate the estrous cycle to better fit certain management practices, to terminate pregnancies resulting from mismatings, and to treat certain conditions associated with prolonged luteal function.RECOMMENDED USES:
Unobserved or Non-detected EstrusCows which are not detected in estrus, although ovarian cyclicity continues, can be treated with estroPLAN if a mature corpus luteum is present. Estrus is expected to occur 2 to 5 days following injection, at which time animals may be inseminated. Treated cattle should be inseminated at the usual time following detection of estrus. If estrus detection is not desirable or possible, treated animals may be inseminated twice at about 72 and 96 hours postinjection.
Pyometra Or Chronic Endometritis
Damage to the reproductive tract at calving or postpartum retention of the placenta often leads to infection and inflammation of the uterus (endometritis). Under certain circumstances, this may progress into chronic endometritis with the uterus becoming distended with purulent matter. This condition, commonly referred to as pyometra, is characterized by a lack of cyclical estrus behavior and the presence of a persistent corpus luteum. Induction of luteolysis with estroPLAN usually results in evacuation of the uterus and a return to normal cyclical activity within 14 days after treatment. After 14 days post treatment, recovery rate of treated animals will not be different than that of untreated cattle.
Mummified Fetus
Death of the conceptus during gestation may be followed by its degeneration and dehydration. Induction of luteolysis with estroPLAN usually results in expulsion of the mummified fetus from the uterus. (Manual assistance may be necessary to remove the fetus from the vagina.) Normal cyclical activity usually follows.
Luteal Cysts
A cow may be noncyclic due to the presence of a luteal cyst (a single, anovulatory follicle with a thickened wall which is accompanied by no external signs and by no changes in palpable consistency of the uterus). Treatment with estroPLAN can restore normal ovarian activity by causing regression of the luteal cyst.
Pregnancies From Mismating
Unwanted pregnancies can be safely and efficiently terminated from 1 week after mating until about 5 months of gestation. The induced abortion is normally uncomplicated and the fetus and placenta are usually expelled about 4 to 5 days after the injection with the reproductive tract returning to normal soon after the abortion. The ability of estroPLAN to induce abortion decreases beyond the fifth month of gestation while the risk of dystocia and its consequences increases. estroPLAN has not been sufficiently tested under feedlot conditions; therefore recommendations cannot be made for its use in heifers placed in feedlots.
Controlled Breeding
The luteolytic action of estroPLAN can be utilized to schedule estrus and ovulation for an individual cycling animal or a group of animals. This allows control of the time at which cycling cows or heifers can be bred. estroPLAN can be incorporated into a controlled breeding program by the following methods:1. Single estroPLAN Injection
Only animals with a mature corpus luteum should be treated to obtain maximum response to the single injection. However, not all cycling cattle should be treated since a mature corpus luteum is present for only 11 to 12 days of the 21-day cycle.
Prior to treatment, cattle should be examined rectally and found to be anatomically normal, be non-pregnant and have a mature corpus luteum. If these criteria are met, estrus is expected to occur 2 to 5 days following injection, at which time animals may be inseminated. Treated cattle should be inseminated at the usual time following detection of estrus. If estrus detection is not desirable or possible, treated animals may be inseminated either once at about 72 hours or twice at about 72 and 96 hours postinjection.
With a single injection program, it may be desirable to assess the cyclicity status of the herd before estroPLAN treatment. This can be accomplished by heat detecting and breeding at the usual time following detection of estrus for a 6-day period, all prior to injection. If by the sixth day the cyclicity status appears normal (approximately 25 - 30% detected in estrus), all cattle not already inseminated should be palpated for normality, non-pregnancy, and cyclicity, then injected with estroPLAN. Breeding should then be continued at the usual time following signs of estrus on the seventh and eighth day. On the ninth and tenth day breeding may continue at the usual time following detection of estrus or all cattle not already inseminated may be bred either once on the ninth day (at about 72 hours post injection) or on both the ninth and tenth day (at about 72 and 96 hours postinjection).
2. Double estroPLAN Injections
Prior to treatment, cattle should be examined rectally and found to be anatomically normal, non-pregnant, and cycling (the presence of a mature corpus luteum is not necessary when the first injection of a double injection regimen is given). A second injection should be given 11 days after the first injection. In normal, cycling cattle, estrus is expected 2 to 5 days following the second injection. Treated cattle should be inseminated at the usual time following detection of estrus. If estrus detection is not desirable or possible, treated animals may be inseminated either once at about 72 hours or twice at about 72 and 96 hours following the second estroPLAN injection.
Many animals will come into estrus following the first injection; these animals can be inseminated at the usual time following detected estrus. Animals not inseminated should receive a second injection 11 days after the first injection. Animals receiving both injections may be inseminated at the usual time following detection of estrus or may be inseminated either once at about 72 hours or twice at about 72 and 96 hours post second injection.
Any controlled breeding program recommended should be completed by either:
- Observing animals (especially during the third week after injection) and inseminating or hand mating any animals returning to estrus,
or
- Turning in clean-up bull(s) 5 to 7 days after the last injection of estroPLAN to cover any animals returning to estrus.REQUIREMENTS FOR CONTROLLED BREEDING PROGRAMS:
A variety of programs can be designed to best meet the needs of individual management systems. A controlled breeding program should be selected which is appropriate for the existing circumstances and management practices.
Before a controlled breeding program is planned, the producer’s objectives must be examined and he must be made aware of the projected results and limitations. The producer and his consulting veterinarian should review the operation’s breeding history, herd health and nutritional status and agree that a controlled breeding program is practical in the producer’s specific situation. For any successful controlled breeding program:
- cows and heifers must be normal, nonpregnant, and cycling (rectal palpation should be performed).
- cattle must be in a fit and thrifty breeding condition and on an adequate or increasing plane of nutrition.
- proper program planning and record keeping are essential.
- if artificial insemination is used, it must be performed by competent inseminators using high quality semen.It is important to understand that estroPLAN is effective only in animals with a mature corpus luteum (ovulation must have occurred at least 5 days prior to treatment). This must be considered when breeding is intended following a single estroPLAN injection.
SAFETY AND TOXICITY:
At 50 and 100 times the recommended dose, mild side effects may be detected in some cattle. These include increased uneasiness, slight frothing, and milk let-down.CONTRAINDICATIONS:
estroPLAN should not be administered to a pregnant animal whose calf is not to be aborted.WARNINGS:
For animal use only.Women of child-bearing age, asthmatics, and persons with bronchial and other respiratory problems should exercise extreme caution when handling this product. In the early stages women may be unaware of their pregnancies.
estroPLAN injection is readily absorbed through the skin and may cause abortion and/or bronchospasms; direct contact with the skin should therefore be avoided. Accidental spillage on the skin should be washed off immediately with soap and water.
PRECAUTIONS:
There is no effect on fertility following the single or double dosage regimen when breeding occurs at induced estrus or at 72 and 96 hours post treatment. Conception rates may be lower than expected in those fixed time breeding programs which omit the second insemination (i.e. the insemination at or near 96 hours). This is especially true if a fixed time insemination is used following a single estroPLAN injection.As with all parenteral products, careful aseptic techniques should be employed to decrease the possibility of postinjection bacterial infection. Antibiotic therapy should be employed at the first sign of infection.
The Material Safety Data Sheet (MSDS) contains more detailed occupational safety information. To obtain an MSDS or for technical assistance, contact Parnell at 1-800-88-PARNELL (1-800-887-2763). To report suspected adverse drug experiences, contact Parnell at 1-800-88-PARNELL (1-800-887-2763). For additional information about adverse drug experience reporting for animal drugs, contact FDA at 1-888-FDA-VETS, or http://www.fda.gov/reportanimalae.
DOSAGE AND ADMINISTRATION:
Two mL of estroPLAN injection (500 mcg of cloprostenol) should be administered by INTRAMUSCULAR INJECTION for all indications in both beef and dairy cattle.Discard remaining product 180 days after first use.
STORAGE CONDITIONS:
1. Protect from light.
2. Store in carton.
3. Store at controlled room temperature 20°-25°C (68°-77°F).HOW SUPPLIED:
20 mL and 100 mL multidose vials
Made in Australia
Manufactured by:
PARNELL TECHNOLOGIES PTY. LTD.
4/476 Gardeners Road
Alexandria NSW 2015 Australia
Owner of the registered trademark estroPLAN®
Distributed by:
PARNELL U.S. 1, Inc.
7015 College Boulevard
Level 6
Overland Park, KS 66211
Approved by FDA under ANADA # 200-310
20mL: 50299b-07-June 20
100mL: 50301b-06-June 20 -
PRINCIPAL DISPLAY PANEL - 20 mL Vial
estroPLAN®
(cloprostenol sodium)
Equivalent to 250 mcg cloprostenol/mL
CAUTION: Federal law restricts this drug to use by or on the order of a licensed veterinarian.
20 mL 10 doses
Approved by FDA under ANADA # 200-310
PARNELL TECHNOLOGIES PTY. LTD.
4/476 Gardeners Road, Alexandria NSW 2015, Australia
20 mL

-
Principal Display Panel - 20 mL Carton
estroPLAN®
(cloprostenol sodium)
Equivalent to 250 mcg cloprostenol/mL
An analogue of prostagandin F2α for intramuscular injection in beef and dairy cattle.
20 mL 10 doses
Approved by FDA under ANADA # 200-310
CAUTION: Federal law restricts this drug to use by or on the order of a licensed veterinarian.
FOR ANIMAL USE ONLY
PARNELL
living SCIENCE
20 mL

-
Principal Display Panel - 100 mL Vial
estroPLAN®
(cloprostenol sodium)
Equivalent to 250 mcg cloprostenol/mL
CAUTION: Federal law restricts this drug to use by or on the order of a licensed veterinarian.
100 mL 50 doses
ANADA 200-310, Approved by FDA
PARNELL TECHNOLOGIES PTY. LTD.
4/476 Gardeners Road, Alexandria NSW 2015, Australia
100 mL

-
Principal Display Panel - 100 mL Carton
estroPLAN®
(cloprostenol sodium)
Equivalent to 250 mcg cloprostenol/mL
An analogue of prostagandin F2α for intramuscular injection in beef and dairy cattle.
100 mL 50 doses
Approved by FDA under ANADA # 200-310.
CAUTION: Federal law restricts this drug to use by or on the order of a licensed veterinarian.
FOR ANIMAL USE ONLY
PARNELL
living SCIENCE
100 mL

-
INGREDIENTS AND APPEARANCE
ESTROPLAN
cloprostenol sodium injection, solutionProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC:68504-001 Route of Administration INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength cloprostenol sodium (UNII: 886SAV9675) (cloprostenol - UNII:4208238832) cloprostenol 250 ug in 1 mL Inactive Ingredients Ingredient Name Strength CHLOROCRESOL (UNII: 36W53O7109) 1 mg in 1 mL ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) 0.66 mg in 1 mL sodium citrate (UNII: 1Q73Q2JULR) 5.03 mg in 1 mL sodium chloride (UNII: 451W47IQ8X) 6.76 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68504-001-01 1 in 1 CARTON 1 20 mL in 1 VIAL, MULTI-DOSE 2 NDC:68504-001-02 1 in 1 CARTON 2 100 mL in 1 VIAL, MULTI-DOSE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANADA ANADA200310 03/01/2013 Labeler - Parnell Technologies Pty Ltd (742511504)




