DR. COCOA DAYTIME COUGH AND COLD- dextromethorphan hydrobromide and phenylephrine hydrochloride liquid
Pernix Therapeutics, LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Dr. Cocoa Daytime Cough+Cold
Uses
temporarily relieves:
- cough due to minor throat and bronchial irritation as may occur with a cold
- nasal congestion
Warnings
Do not use if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
- heart disease
- high blood pressure
- diabetes
- thyroid disease
- trouble urinating due to an enlarged prostate gland
- persistent or chronic cough such as occurs with smoking, asthma, or emphysema
- cough that occurs with too much phlegm (mucus)
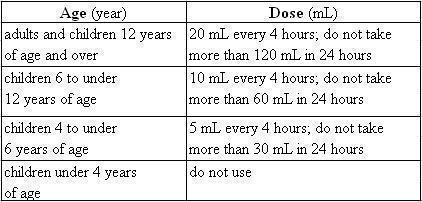
Directions
- shake well before use
- measure only with dosing spoon provided
- read dosing spoon carefully. Dosing spoon holds 5 mL when filled to the top rim
- do not use dosing spoon with other products
- dose as follows or as directed by a doctor
- mL = milliliter
Inactive ingredients
anhydrous citric acid, cocoa, maltitol, methylparaben, natural flavor, propylene glycol, purified water, sodium benzoate, sorbitol, sucralose, trisodium citrate dihydrate
| DR. COCOA DAYTIME COUGH AND COLD
dextromethorphan hydrobromide, phenylephrine hydrochloride liquid |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Labeler - Pernix Therapeutics, LLC (004672296) |

