Label: ZN7 DERM- zinc gluconate gel
- NDC Code(s): 86045-6000-1, 86045-6000-2
- Packager: Addison Biological Laboratory, Inc.
- Category: OTC ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated November 2, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- INDICATIONS & USAGE
- INSTRUCTIONS FOR USE
- SPL UNCLASSIFIED SECTION
- PRECAUTIONS
- SPL UNCLASSIFIED SECTION
-
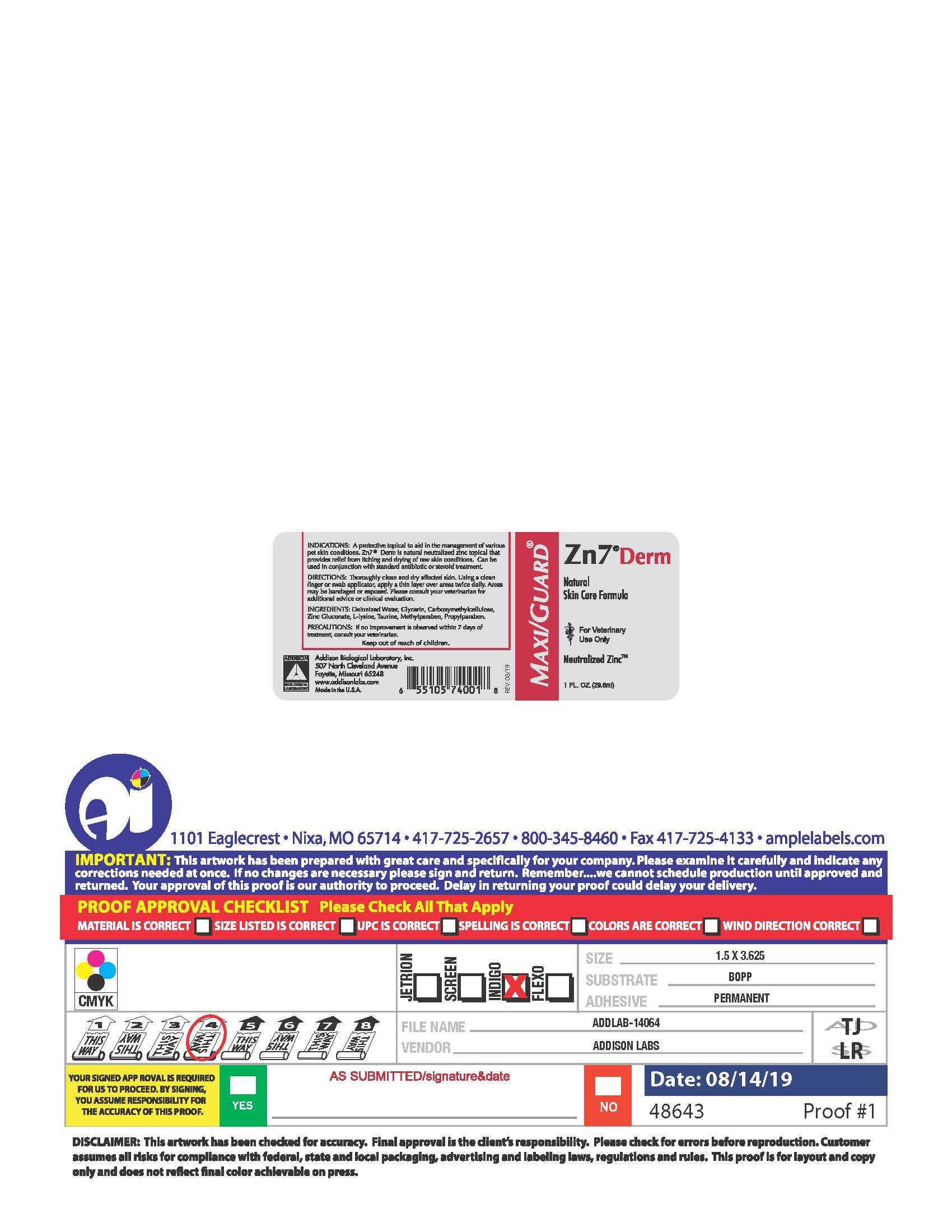
PRINCIPAL DISPLAY PANEL
MAXI/GUARD®
Zn7®Derm
Natural
Skin Care Formula
For Veterinary
Use Only
Neutralized Zinc™
1 FL. OZ. (29.5 ml)
INDICATIONS: A protective topical to aid in the management of various pet skin conditions. Zn7®Derm is natural neutralized zinc topical that provides relief from itching and drying of raw skin conditions. Can be used in conjunction with standard antibiotic or steroid treatment.
DIRECTIONS: Thoroughly clean and dry affected skin. Using a clean finger or swab applicator, apply a thin layer over the areas twice daily. Areas may be bandaged or exposed. Please consult your veterinarian for additional advice or clinical evaluation.
INGREDIENTS: Deionized Water, Glycerin, Carboxymethycellulose, Zinc Gluconate, L-Lysine, Taurine, Methylparaben, Propylparaben.
PRECAUTIONS: If no improvement is observed within 7 days of treatment, consult your veterinarian.
Keep out of reach of children.
Addison Biological Laboratory, Inc.
507 North Cleveland Avenue
Fayette, Missouri 65248
www.addisonlabs.com
MADE IN THE U.S.A.
Rev. 08/19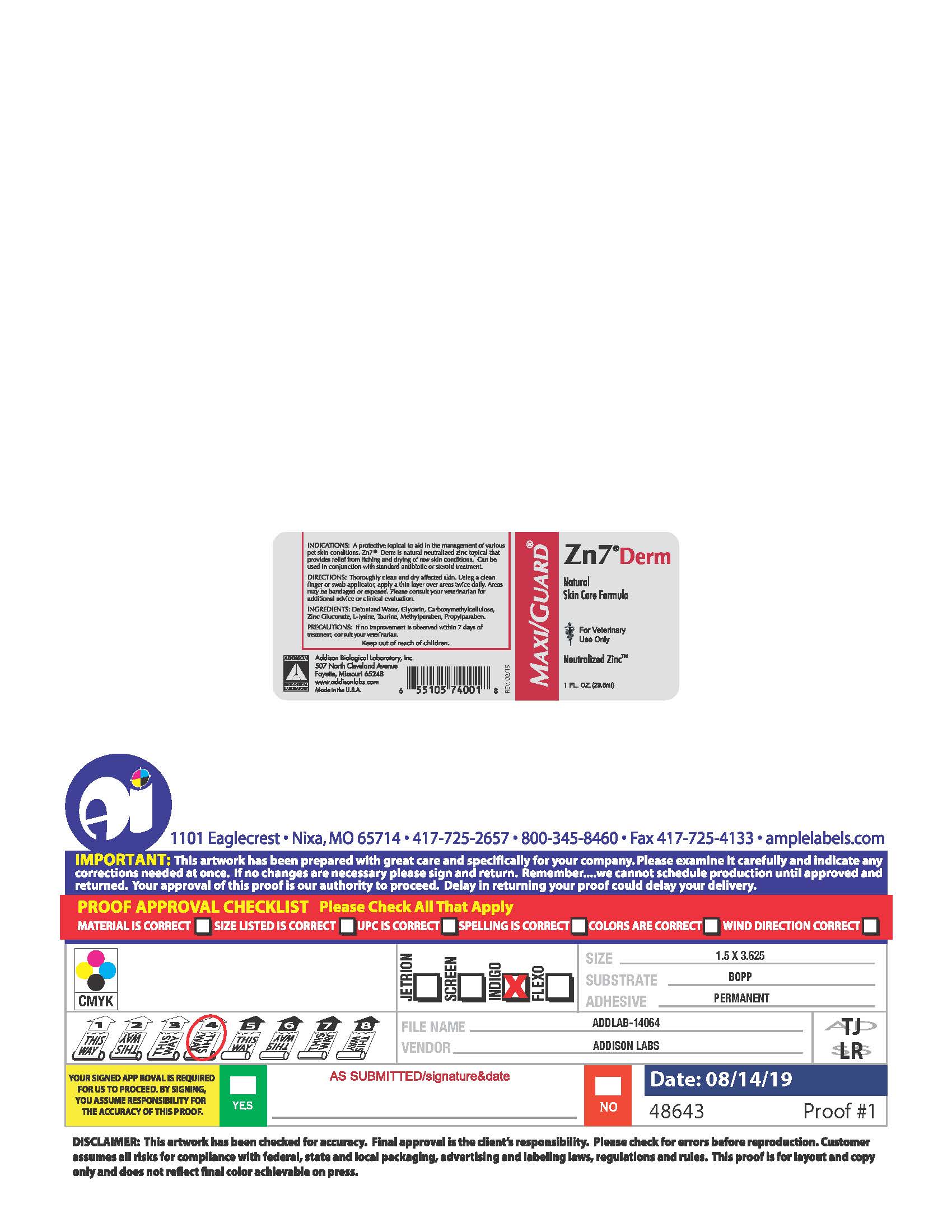
-
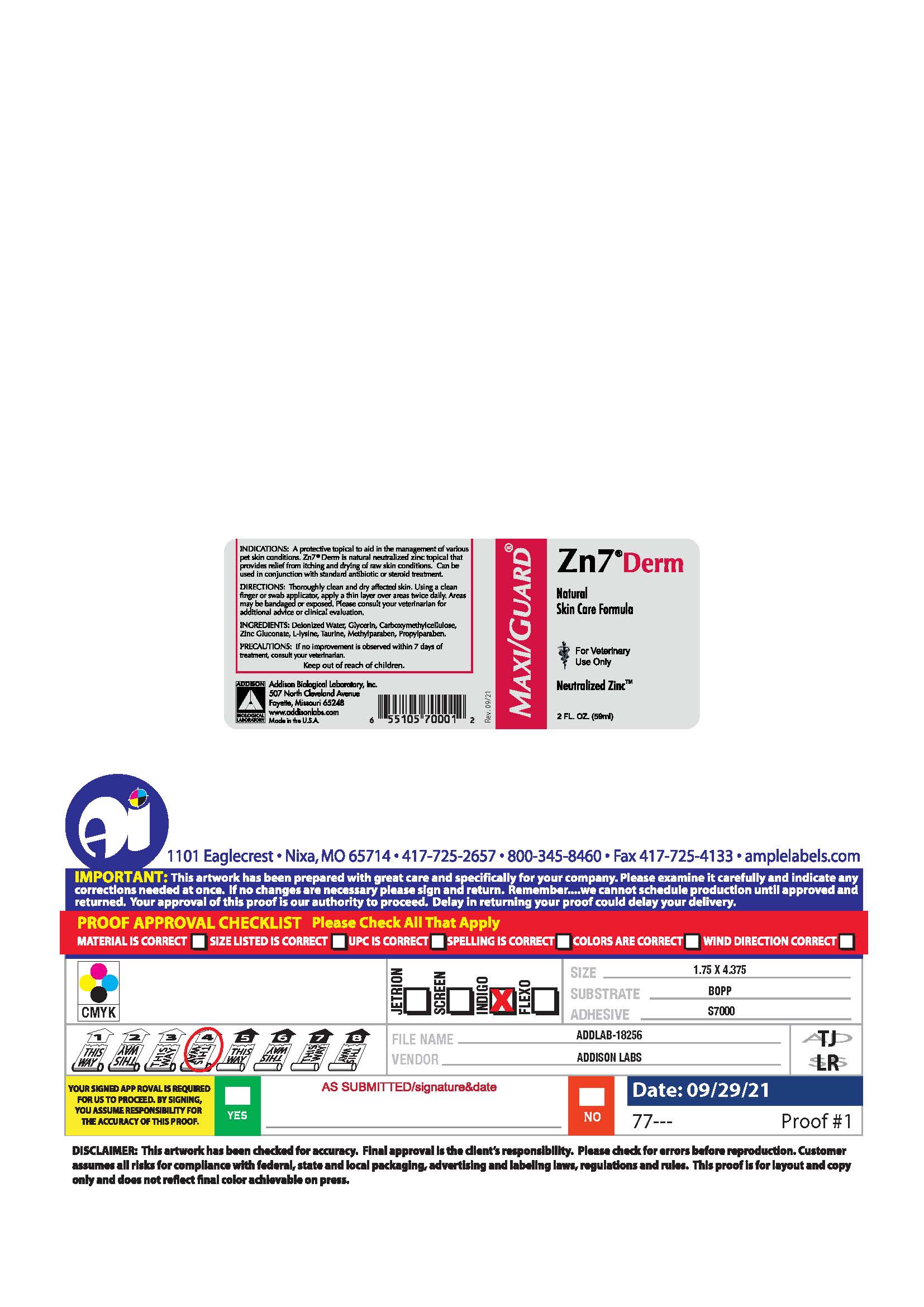
PRINCIPAL DISPLAY PANEL
MAXI/GUARD®
Zn7®Derm
Natural
Skin Care Formula
For Veternary
Use Only
Neutralized Zinc™
2 FL. OZ. (59 ml)
INDICATIONS: A protective topical to aid in the management of various pet skin conditions. Zn7®Derm is natural neutralized zinc topical that provides relief from itching and drying of raw skin conditions. Can be used in conjunction with standard antibiotic or steroid treatment.
DIRECTIONS: Thoroughly clean and dry affected skin. Using a clean finger or swab applicator, apply a thin layer over the areas twice daily. Areas may be bandaged or exposed. Please consult your veterinarian for additional advice or clinical evaluation.
INGREDIENTS: Deionized Water, Glycerin, Carboxymethycellulose, Zinc Gluconate, L-Lysine, Taurine, Methylparaben, Propylparaben.
PRECAUTIONS: If no improvement is observed within 7 days of treatment, consult your veterinarian.
Keep out of reach of children.
Addison Biological Laboratory, Inc.
507 North Cleveland Avenue
Fayette, Missouri 65248
www.addisonlabs.com
MADE IN THE U.S.A.
Rev. 09/21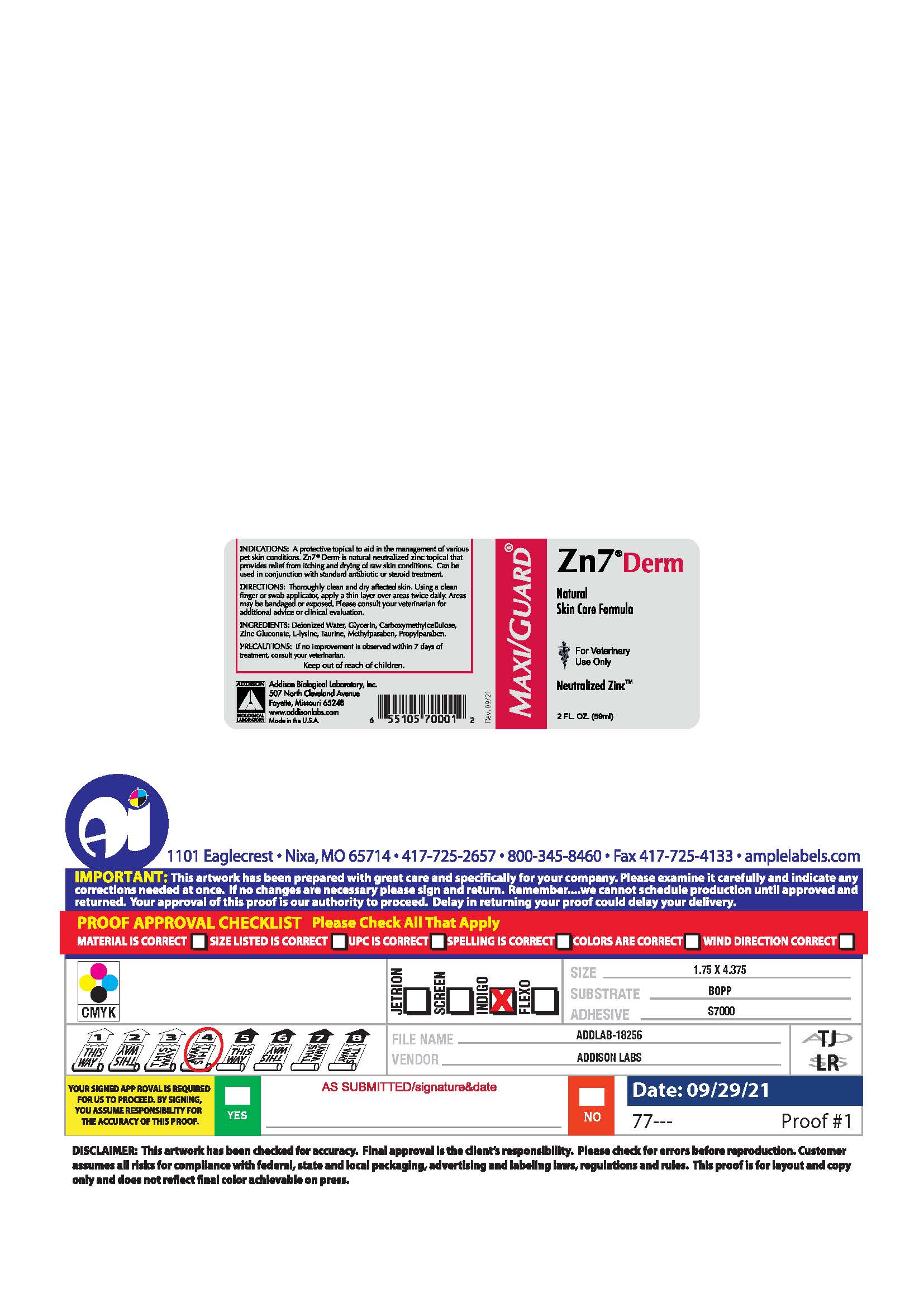
-
INGREDIENTS AND APPEARANCE
ZN7 DERM
zinc gluconate gelProduct Information Product Type OTC ANIMAL DRUG Item Code (Source) NDC:86045-6000 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC GLUCONATE (UNII: U6WSN5SQ1Z) (ZINC CATION - UNII:13S1S8SF37) ZINC GLUCONATE .011 g in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) TAURINE (UNII: 1EQV5MLY3D) METHYLPARABEN (UNII: A2I8C7HI9T) PROPYLPARABEN (UNII: Z8IX2SC1OH) LYSINE (UNII: K3Z4F929H6) GLYCERIN (UNII: PDC6A3C0OX) CARBOXYMETHYLCELLULOSE (UNII: 05JZI7B19X) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:86045-6000-1 29.5 mL in 1 PACKAGE 2 NDC:86045-6000-2 59 mL in 1 PACKAGE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 01/01/1998 Labeler - Addison Biological Laboratory, Inc. (118396730) Registrant - Addison Biological Laboratory, Inc. (118396730) Establishment Name Address ID/FEI Business Operations Addison Biological Laboratory, Inc. 118396730 manufacture Establishment Name Address ID/FEI Business Operations Jost Chemical Co. 147882294 api manufacture Establishment Name Address ID/FEI Business Operations ADAMSON ANALYTICAL LABORATORIES INC 944399906 analysis Establishment Name Address ID/FEI Business Operations Alliance Analytical Laboratories, Inc. 007588338 analysis